I’m preparing a series of brief lectures on aspects of coronavirus biology. Here are the first two:
Category Archives: Epidemics
An epidemic of fear
How does fear of epidemic disease influence the spread of disease? In the movie “Contagion” we see many examples of how individuals and governments respond to an epidemic. There is panic, people fleeing cities, people isolating themselves in their homes, governments closing borders and imposing quarantines. The tagline of the movie, “nothing spreads like fear” may actually be quite accurate.
Using mathematical models to understand the spread of disease is a common tool in epidimiology. At its most basic, we can think about the spread of a disease through a population by considering that an individual can be susceptible to the disease, infected, or removed. Someone who is susceptible can become infected. Someone who is infected can spread the disease to others who are susceptible. Infected people can be “removed” from the population by either recovering and becoming immune, or dying. Either way, they are no longer in the susceptible or infected categories. These S-I-R models can become quite complicated when you begin to consider all the possible variations: how easily the disease is transmitted, how long someone is contagious, whether transmission is dependent on direct contact, etc. These models don’t have to be limited to study the spread of infectious disease: indeed, one could consider fear to be contagious.
So what happens to the spread of disease during an epidemic if we add normal human behavior into the SIR model? If we consider fear to be contagious as well, during an epidemic there there would be two things spreading through the population: fear and disease. And when we fear disease we change our behaviour. In a recent paper, the simultaneous spread of fear and disease were modeled using an SIR model. It turns out that, as the tagline for “Contagion” suggests, fear spreads more quickly than disease. In their model, someone susceptible to fear can become “infected” with fear either through contact with someone with the disease, someone who has fear but no disease, or someone who has both fear and disease. Since you can only get infected with the disease by contact with someone else who has the disease, there are more ways to catch fear than disease, and it spreads faster.
So once someone is infected with fear, what do they do? What would you do? My instinct would be to hide at home and remain isolated; others I have spoken to would flee to a small town in the middle of nowhere. The researchers considered that too. How will the spread of the disease change if some people run and some hide and others don’t do anything? It turns out it is best to hide. Hiding has the greatest impact on halting the spread of the disease: not only do fewer people become infected, but the disease doesn’t spread as far geographically. Fleeing reduces the number of people infected (compared to not doing anything) but spreads the disease further.
The model demonstrated another important point. Over time, our fear decays. For whatever reasons, after hiding for a while, we may feel more confident about wandering out into the now hopefully less plague ridden world. Bad idea. As long as there are still some people infected with the actual disease, re-entering the world adds fuel to the epidemic by introducing more susceptibles. This causes a second wave of the epidemic. One could speculate that this could have been the cause of the two waves of influenza in 1918 – people came out of quarantine too early.
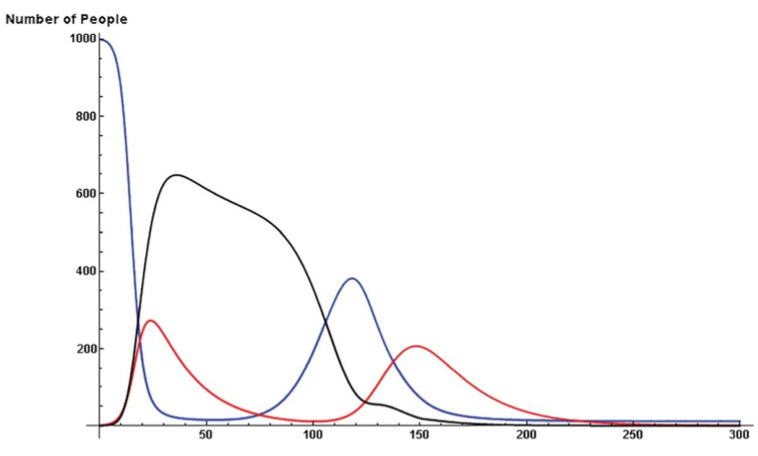
We had a terrific discussion about this paper in class and we identified several reasons why we though that fear would actually spread even faster than the paper suggested, and that the effects of fear (fleeing or hiding) on spread of the disease might be greater. Obviously, fear can spread without direct contact. You can get infected with fear pretty easily by reading things on the internet or watching the news. Fear of a disease could leap across the globe quickly, well ahead of the disease itself. Also, people don’t behave independently. So if one member of a family becomes afraid and wants to flee or hide, the rest of the family may do so as well. As people flee from cities, its likely that choke-points and crowds would further fuel the epidemic.
So in the event of a massive, lethal epidemic, would the politicians and army have the willpower to impose a long enough population-wide quarantine and prevent people from fleeing an infected city? Would President Obama/Romney say “No really, everyone must stay at home, see there’s this paper in PLoS ONE by Epstein et al…”
Reference:
H5N1 Ferret Transmission Experiment Published
At last, one of the papers investigating H5N1 influenza transmission in ferrets has been published in the journal Nature yesterday. To recap the controversy briefly: news of experimental studies investigating transmissibility of avian H5N1 influenza hit the news this fall, igniting a fierce debate about biosecurity, “dual use” research, and the damage that censorship can have on scientific advancement. An advisory group, the NSABB, recommended partial censorship of the data, perhaps believing that redacting specific data from the publications would prevent information on how to generate a highly pathogenic mammalian transmissible virus from getting into the hands of bioterrorists or others incapable of handling such viruses safely. However, after some new data or clarification of data presented in a revised version of the submitted manuscript, the NSABB recommended publication in full.
Avian H5N1 influenza virus has caused sporadic infections in humans who have close contact with infected animals. Human-to-human transmission has not been observed. But could an H5 virus mutate or reassort, allowing human-to-human transmission?
One thing that has been lost in this whole controversy is that this study is actually a great demonstration and application of evolution. How does a virus switch to a new host and transmit efficiently between individuals? Start with a diverse population that varies in a particular trait (in this case, ability to bind the human receptor). Put it through selective pressure. This occurred in several steps: first, select for viruses that can bind the human receptor in vitro. From those that bind, select ones that can efficiently replicate in the respiratory tract of the animal. Finally, take the efficient replicators and allow for transmission. At each step of selection, mutations that naturally occur during viral replication further diversify the population resulting in variants that possess the desired property. Those variants get selected for the next experiment.
This study focused on one particular influenza virus protein, hemagglutinin (HA). HA is on the surface of the virion and is what the virus uses to attach to the host cell, the first step in viral infection. HA of human influenza viruses bind a sugar on the surface of human cells, which is slightly different from that found in the avian respiratory tract. Avian viruses, of course, bind to the form found in birds. H5 shows a strong preference for binding the avian receptor, so Karaoka et al were interested in finding out if H5 could change to recognize the human receptor, allowing more efficient transmission between mammals.
To address this, they began with a mutagenesis technique to introduce random mutations in the globular head (the receptor binding part) of HA, then used an in vitro approach to select for mutants that bound the human receptor (selection step 1). Through this process they identified three H5 variants that gained the ability to bind the human receptor while maintaining the ability to bind the avian receptor, and one that switched specificity completely to the human receptor. Several of the mutations identified in this study had already been shown in previous studies to be important in receptor specificity.
To test if the variant H5s conferred binding to human receptors in vivo, sections of human tracheal tissue were exposed to the viruses and only two were able to infect (selection step 2). This suggests the virus can infect human epithelium of the upper respiratory tract.
Next, the two remaining variants were used to infect ferrets. Both replicated in ferret respiratory tracts, but one replicated to higher levels. When they sequenced the virus that they isolated from that ferret, it was different from what they had put in: a new mutation had appeared. This new mutation presumably confers the property of better replication in the ferret respiratory tract, so it outgrew the original input virus (selection step 3).
Using this new virus (now with a total of 3 mutations in H5), transmission was tested in ferrets. Compared to the original H5, which did not transmit via aerosol, the 3-mutation variant did transmit, although between only 2 of 6 animal pairs. Again, they sequenced the virus that was present in the contact animals and found that it was different than what had gone in to the inoculated animals. Yet another mutation had appeared (selection step 4). This additional mutation appears to enhance transmission: the new virus, now with 4 mutations, transmitted more quickly and between more pairs of animals than the 3-mutation virus. Although the virus can transmit, none of the infected animals died, but they did show pathology at the site of infection.
So what does this all mean? The best model available for influenza transmission studies is ferrets. Ferrets aren’t humans, so its important to keep in mind that this is a model that helps us understand what viral or host factors are involved in aerosol transmission in these mammals, and maybe, but not necessarily, in humans. Since we don’t know what is necessary for human-to-human transmission, it is valuable to have an animal model to give us some ideas of what to look for. It can provide some good hypotheses on what mediates transmission in humans, which would then have to be further tested. Obviously, specificity for the human receptor is necessary, but the mutations identified tell us more than that. Mutations that change specificity are not sufficient for transmission. It turns out that those mutations also decrease the stability of the HA protein. The additional mutations acquired through the selection steps compensate for that, and enhance stability. So now we know that HA stability is important in influenza transmission. Between ferrets. That’s probably true for humans too, but it would need to be tested.
If you are still reading, you are obviously procrastinating, and are probably avoiding studying for your final exam. But here are some more thoughts on the controversy overall. This is a really interesting paper, with nothing particularly frightening or worrisome about it. Certainly not any more so than other papers doing similar work that were published without so much controversy. If “dual use” research needs to be regulated, it needs to be done before the work is done, not after. If the NSABB was concerned about this kind of research, why only express concern once the experiment succeeds? In my intro biology class, we read another paper, published in 2005, which was addressing the exact same question, in an almost identical way. The difference is that they failed to make a transmissible virus. If there is a concern about this kind of research, a concern that it is too risky to do these kinds of experiments, shouldn’t the alarm have been raised regardless of the outcome? It just doesn’t make sense to me why it suddenly became so concerning. If anything good has come of this controversy, it is the widespread discussion that this has stimulated on the importance of open communication of scientific data, the importance of not censoring in science. Ironically, had we all been given access to the data, like through a journal publication, it would have been apparent that there wasn’t anything to be concerned about.
Luring HIV out of its latency may be the secret to developing an effective HIV cure
Contributed by guest blogger: Steven Chan ‘12
The emergence of highly active antiretroviral therapy (HAART) in the treatment of HIV-infected individuals has certainly changed the outlook of an HIV diagnosis today, compared to what such an outlook looked like in the earliest years of the epidemic. Such a treatment regimen, if strictly adhered to, has the potential to suppress the levels of active circulating HIV in the infected individual to a level that is manageable, essentially halting the progression of the disease. It soon became clear however, that these treatments could not effectively clear the body of all HIV particles—the virus manages to stow itself away within the cellular genome of the memory CD4+ T-cells, and remain transcriptionally silent indefinitely. These latent reservoirs of HIV-infected cells prove to be undetectable for these antiretroviral therapies, since antiretroviral drugs can only target HIV-infected cells when they are replicating. And so, memory cells, which replicate infrequently, cannot be effectively targeted, making it impossible to clear HIV-infected bodies of all HIV-particles. “We’re never going to cure anybody unless we go for this latent pool,” says Robert Siliciano, the researcher at Johns Hopkins University that first identified the latent HIV memory-T cells.
A great deal of HIV-therapy research over the past decade has focused on finding a way to coax these infected cells out of their latency to make them detectable by antiretroviral drugs. The problem that has been persistently hounding researchers has been the difficulty in luring these cells out of their latency without triggering the immune system in an inflammation response that would end up doing more harm than good. David Margolis, MD, and his research team at UNC Chapel Hill, who have been working on this problem for a while now, have found success with a set of histone deacetylase inhibitors called Zolinza (vorinostat), a chemotherapeutic cancer drug that has been found to stimulate gene expression within the latent HIV-infected cells without inducing an overwhelming immune response. HDAC inhibitors accomplish this by inhibiting the activity of histone deacetylase, which removes the acetyl groups from the lysine residues in the core histones, resulting in the formation of a condensed and transcriptionally silenced chromatin. By inhibiting this activity, the core histones become less compact, and the chromatin becomes more transcriptionally active. After initial success with in vitro tests in cell cultures and in blood tissues, six HIV-positive men were recruited in a clinical trial pairing this treatment alongside consistent antiretroviral therapy. Each of the study volunteers had already been taking part in a robust antiviral regimen for an average of four years, and displayed undetectable viral loads and stable CD4+ T-cell counts. Post-exposure to Zolinza, HIV-RNA levels—a marker of viral activity—in these patients increased by an average of 4.8 times, ranging from a 1.5-fold increase in one patient to a 10.0-fold increase in another. The drug took effect in as little as 8 hours, inducing a two-fold increase in cellular and chromatin-bound histone acetylation within that time span. Increased expression made these cells susceptible to detection and eradication by the antiretroviral drugs, which proceeds just as efficiently as usual.
Margolis addresses the significance of this advancement, “This study provides first proof of concept, demonstrating disruption of latency, a significant step toward eradication.” Just how effective this drug is in teasing out the latent cells still remains to be seen—with nearly a ten-fold difference in one trial participant compared to the other, the efficacy of such a drug remains questionable. The limited sample size in this initial trial also doesn’t give us too much to go on. There are also concerns that the drug could induce some serious side effects such as blood clots in the legs and lungs, diabetes, fewer platelets and RBC count, as well as dehydration from nausea and vomiting, but at least in this trial, there were only mild adverse effects at worst. Little is known about the potential adverse effects of long-term use of the drug. Margolis et al.’s study design made use of a single dose of Vorinostat, but it is likely that repeated intermittent doses would yield the most optimal effects. “Vorinostat may not be the magic bullet, but this success shows us a new way to test drugs to target latency and suggests that we can build a path that may lead to a cure,” says Margolis. Further studies to assess Vorinostat’s safety and effectiveness, and the way it interacts with other HAART treatments, would certainly be crucial before it can be deployed as a component in future HIV treatment regimen.
Links:
Archin N, Liberty A, Kashuba A, Choudhary S, Kuruc J, Hudgens M, Kearney M, Eron J, Hazuda D, and Margolis D. “Administration of Vorinostat Disrupts HIV-1 Latency in Patients on ART,” HIV Persistence, Latency, and Eradication at 19th Conference on Retroviruses and Opportunistic Infections, March 8, 2012, http://www.retroconference.org/2012b/Abstracts/45315.htm
Contreras X, Schwenwker M, Chen CS, McCune JM, Deeks SG, Martin J, Peterlin BM. Suberoylanilide Hydroxamic Acid Reactivates HIV from Latently Infected Cells, J. Biol. Chem., January 9, 2009, http://www.jbc.org/content/284/11/6782.full
Horn T. “Pathway to a Cure: Cancer Drug Helps Purge HIV From Resting Cells,” AidsMeds, March 9, 2012, http://www.aidsmeds.com/articles/hiv_vorinostat_ cure_1667_22059.shtml
“Lymphoma Drug Wakes Up Dormant HIV,” AidsMeds, March 17, 2009, http://www.aidsmeds.com/articles/hiv_zolinza_latent_1667_16307.shtml
Steven Chan is a senior at Vassar College, majoring in Science, Technology, and Society
Highly Active Antiretroviral Therapy and Tenofovir: Lowering HIV Viral Loads, Raising the Risk of Renal Failure
Contributed by guest blogger: Michael McManus ‘12
People undergoing anti-retroviral therapies, which target and interrupt the replicative processes of HIV, are living longer due to the relative success of treatments. Those with HIV are using these drug cocktails for longer periods of time, an important characteristic with results that could not be observed in short-term clinical studies.
Mortality for patients with HIV who are able to undergo highly active antiretroviral therapy (HAART) has shifted from higher rates, during the initial HIV scare, to relatively lower rates. HAART has been incredibly successful, increasing the quality of life for those who have access to it. For some, however, the effects of an HAART regimen, which combines up to four medications, can lead to renal failure within two weeks of regimen administration due to the toxic nature of some of the medications.
Renal failure, or loss of kidney function, can lead to organ failure and eventually death. The kidneys are normally responsible for filtering the blood. They maintain homeostasis by regulating electrolytes, regulating blood pressure, maintaining pH balances, and by removing and diverting waste from the blood into the urinary bladder, producing urine. The kidneys filter many things from the blood in order to retain them in the body, ranging from proteins to glucose. When the kidneys fail, proteins, glucose, and even blood become detectable in the urine. Glycosuria, proteinuria, and hematuria are all biological indicators of a lack of reabsorption and therefore renal failure.
In a recently published paper, Juliette Pavie et al. describe a case of renal failure in an HIV-positive patient after only two weeks of tenofovir therapy. Tenofovir is a nucleotide reverse transcriptase inhibitor associated with low risk of severe renal adverse events in clinical trials. However, tenofovir is in the same class of drugs such as adefovir and cidofovir, which have well-documented nephrotoxicity1,2.
The patient followed was a 46-year-old homosexual male of Scottish descent. His weight, CD4 cell count, plasma HIV RNA level, serum creatine, and urea levels were all taken before HAART regimen, which consisted of tenofovir, emtricitabine, atazanavir, and ritonavir, was started. During treatment, the patient did not take any nonsteroidal anti-inflammatory drugs (NSAIDs), which are known to lead to kidney dysfunction.
Fifteen days after treatment began levels were tested again: serum creatine and urea had increased 3-fold and 2.5-fold respectively. Five days later, the patient became unable to pass urine and levels were immediately measured again: serum creatine showed a 15-fold increase from the original amount, and urea a 12-fold increase. Urinalysis showed glycosuria and proteinuria, indicating loss of kidney function. Renal biopsy indicated necrosis of the kidneys and other abnormalities. After these observations, HAART was halted and hemodialysis was started to rescue lost kidney function. After three months of hemodialysis, HAART was resumed, but tenofovir was excluded from the treatment.
After one year of treatment, the patient showed signs of recovery. CD4 cell count increased to relatively normal levels, and serum creatine and plasma HIV RNA levels dropped. To this day, the patient is still undergoing the same HAART, with serum creatine and plasma HIV RNA levels remaining stable. However, the patient still suffers from moderate glycosuria and proteinuria, indicating that kidney function has not fully recovered.
Although this case only followed one individual undergoing a HAART regimen containing tenofovir, the observations and results are still crucial to studying renal failure resulting from HAART. The novel form of nephrotoxicity observed may serve as a model for other forms of nephrotoxicity caused by reverse transcriptase inhibitors. Although the nephrotoxicity studies1,2 only reported findings in one individual each, their findings should not be discredited, as this is the nature HIV symptom studies. For example, the emergence of Kaposi’s sarcoma as a symptom of HIV began with isolated incidents. The nature of HIV and its rapid mutation also obfuscates the relationship between HAART effectiveness and strain type. From this observation, one question out of many must be addressed: Did the combination of drugs used in the patient’s HAART regimen have an effect on nephrotoxicity?
Despite the emergence of renal failure as a threat, great strides have been made in the fight against HIV. The quality of life for those who are suffering from HIV and who have access to HAART is drastically improved compared to those who are unable to undergo HAART. However, now that HIV patients are living longer, research must switch from just targeting HIV to focusing on HIV and the complications created by decreased mortality. Nephropathy, or disease of the kidney, and subsequent nephrectomy, removal of a kidney, now contribute to the decrease in quality of life associated with the aging HIV population. When developing future treatments, scientists and doctors must analyze the nephrotoxicity of the products they are synthesizing, as renal failure is a clear and present danger for those undergoing HAART.
Article Links:
http://online.liebertpub.com/doi/abs/10.1089/apc.2011.0056
Michael McManus is a senior at Vassar College, majoring in Biochemistry
HIV Microbicides and the Risks of Clinical Trials
Contributed by guest blogger: Julia Ding ’12
Once preliminary studies suggest that a drug is safe for human use, clinical trials are conducted in order to further investigate the effects and possible adverse reactions of the drug. The example of HIV microbicides has shown that caution and careful scrutiny is highly important for these trials. HIV microbicides are chemical entities which, when applied before vaginal or rectal intercourse, prevent the transmission of the virus. Of the potential microbicide agents that have been studied, two compounds classified as polyanions were thought to be promising for inhibiting HIV-1 transmission: carrageenan and cellulose sulfate (CS). However, these compounds were deemed in phase III clinical trials to be ineffective as microbicides.
In addition to that discovery, the more surprising and disturbing result of these trials was that the HIV microbicides appeared to actually enhance the rates of HIV infection. Pirrone and colleagues examined the validity of this claim in a study reassessing the in vitro activities of the compounds. Cells were infected with different strains of HIV-1 in the presence of three different polyanions: CS, λ-carrageenan (LC), and destran sulfate (DS). Resulting assays showed that all of these compounds exhibited antiviral activity against both R5 and X4 HIV-1 strains. However, further experiments also discovered that application and removal of polyanion microbicides prior to HIV exposure enhanced and increased the rates of HIV-1 infection. The compounds were added to cell cultures and washed out prior to HIV-1 infection to simulate the natural loss of the compound after vaginal application. In both HIV-susceptible cells and regular human cells, the results indicated an increase in the percentage of cells infected, unrelated to any change in cell viability. The level of enhancement was found to be dependent on the target cell, its co-receptor phenotype, the compound identity and concentration, and the timing of the viral challenge. While the mechanism through which HIV-1 transmission increased in the in vitro experiments is still unclear, these factors suggest that the nature of the host cell also plays a role in polyanion-dependent HIV-1 infection. This data provides a discouraging outlook on the use of these compounds as effective microbicides, while introducing new questions about its mechanisms of action.
This study provides us with many valuable insights about not only the microbicide technology itself, but also the risks and complications associated with clinical trials. The data suggested a significant increase in HIV-1 infection after the application and removal of the two microbicides. Furthermore, it emphasized the need for intense scrutiny of compounds prior to clinical trials, considering the dangers they may pose on human subjects. While previous studies supported the use of polyanion microbicides as a safe and possibly effective means of preventing HIV-1 transmission in women, the effects of the leakage and loss of the product over time was not taken into consideration, and significantly more women on the drug were found to have contracted HIV than if they had not taken it. The study also provides us with an example of the vital role clinical trials play in the testing of a drug, and how certain adverse effects may be missed through in vitro studies that only become apparent when applied to real world uses.
Links:
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3295645/
http://www.nejm.org/doi/full/10.1056/NEJMoa0707957#t=abstract
Julia Ding is a senior at Vassar College, with a major in Science, Technology and Society.
Evolving Avian Flu for Enhanced Transmission
Avian influenza (H5N1) infections have about 60% mortality rate. Only around 600 people are known to have been infected, so it is still a very rare but certainly deadly disease. Those infected are individuals who have direct contact with infected birds. Although some cases of human-to-human transmission have been suspected, good evidence for this is lacking. Its an important virus in agriculture too. When H5N1 is identified in domestic birds, the usual response is a massive cull, resulting in millions of birds killed and farmers left in great financial difficulty.
The question of whether such a virus could mutate to cause a human pandemic is an important one. In the history of flu pandemics, only H1, H2, or H3 viruses have been involved. Is it impossible for H5 to cause a pandemic, or has it simply not happened yet? If it could, would it retain the same level of pathogenicity, or in adapting to human-to-human transmission, would it become less virulent?
To answer the question of the possibility of human-to-human transmission, some researchers in the Netherlands performed some experiment to evolve H5N1 to become more transmissible. They infected ferrets, and over several passages (moving the virus from one animal to the next) managed to encourage transmission between ferrets. The virus adapted to transmission between ferrets, changing slightly from the original virus. The changes are minor, only 5 mutations in 2 genes. However, this research has caused some significant concern: they have generated a transmissible form of a highly pathogenic virus. Is this a good idea?
Importantly, the results have not been published so all this information is from news reports and interviews. Few people have seen the data.
This is what scientists call “dual-purpose” research. On the one hand, it can answer important questions. On the other, it can lead to the development of biological weapons, ideas for biological weapons, or seriously bad accidents. The best science writers are having a hard time not sensationalizing this. Even the researcher who did it seems to be playing up the drama. What if it gets out? Millions will die! But is there a real risk from this virus?
Its hard to know the facts without the published data.
How well does the ferret model the pathogenicity and transmissibility in humans? It is commonly used and generally accepted to be quite good, but it seems a stretch to assume a human pandemic can occur based on transmission between ferrets in the lab. We need to be careful not to over-extend the findings of the study (this is especially the case since the data is not available). The experiment presumably shows that the virus can be transmitted between ferrets. It does not demonstrate that this virus can cause a human pandemic.
How pathogenic is the new virus? Does it cause the same disease as the original virus or did the mutations that allow transmissibility also decrease virulence? Maybe it can spread human-to-human, but its not clear how sick they would get. Further, usually when a virus is passaged several times through a different host species, it adapts to that species and results in attenuation in the original host. This has been observed many times, and has even led to the development of several attenuated vaccines.
Related to this, many evolutionary biologists believe that virulence and transmission are closely tied. That is, a virus that is too deadly will cause outbreaks that fizzle out (Ebola is a good example). Viruses that don’t cause enough disease might have a hard time transmitting too (coughing, sneezing or diarrhea are good examples – a little bit of disease helps get the virus out of your bod and into the next one). Paul Ewald argued that the high mortality rate of the 1918 influenza was in part due to the fact that the conditions at the time allowed for a more deadly virus to evolve. Due to WWI, factors such as overcrowding and troop movements may have allowed a highly virulent virus to be successful. Conditions today may not favor a pandemic by a highly virulent virus. So would a transmissible and pathogenic H5N1 cause a major epidemic or would it fizzle out?
There is also the issue of publication. There is debate on whether the research should be published or kept secret. Does publication provide a roadmap for someone who wants to do this for evil purposes to repeat the experiment and create a biological weapon? It seems to me that even without details of the experiment, enough information is already available to repeat it. Withholding publication would also prevent other researchers from understanding and extending the findings. Any benefits from having done the experiments would be significantly less without publication.
I have seen stories like this one before. Several years ago, an highly pathogenic ectromelia virus (causes mousepox, related to the smallpox virus) was made by adding the gene for Interleukin-4. The researchers did not intend to make a highly pathogenic virus, it was rather a surprise to see this effect. There was much debate about whether they should publish, that perhaps this was a roadmap for building a highly pathogenic poxvirus in humans. They published and we have since learned more about the virus, including the observation that the virus does not transmit effectively, and that doing the same thing in other viruses doesn’t have the same effect. The more we know, the better.
I’d make the same argument here. I’d like to see the research published. The information from this study is probably valuable, addresses an important question, and is only one small step in understanding H5N1 influenza.
I’ll take my milk pasteurized, thanks
A recent article in Vassar’s newspaper, The Miscellany News, discussed the Vassar Raw Milk Co-op, which brings unpasteurized milk to Vassar College (Co-op offers raw milk delivery service, Nov 10,2011). The article raises several important questions about raw milk, pasteurization, and sustainable agriculture, but some of the information presented is incorrect. Importantly, Vassar’s Raw Milk Co-op website, to which readers are directed, has a great deal of misleading, incorrect or unsubstantiated information.
In the production and packaging of milk, it can become contaminated at virtually any stage of the process. That contamination, when it is by organisms like E. coli or Listeria, is what causes milkborne illness. Pasteurization, a process of heating milk to reduce the levels of microorganisms present, will kill those contaminating bacteria if they are present. The risk in drinking raw milk is due to the fact that if it does become contaminated, you will be consuming those pathogens. In a farm environment, it is safe to assume that contamination will, at some point, happen. Pasteurization is one check that we have to protect us from that.
The majority of cases of milkborne illness result in diarrhea and/or vomiting. Occasionally the symptoms can be more severe, such as in last week’s outbreak in California, in which five children have become sick. Three have been sent to the hospital with hemolytic uremic syndrome, which can lead to kidney failure. These cases have led to the recall of the organic raw milk, contaminated with E. coil 0157:H7, which has been linked to the outbreak. There are outbreaks associated with pasteurized milk as well, usually due to post-pasteurization contamination, such as at the packaging stage. However, it is estimated that only 1% of people consume raw milk, but from 2000-2007, 75% of outbreaks were associated with contaminated raw milk.
In NY consumers can choose to buy raw milk from a farm if they determine that they are comfortable with the level of risk. But it is also important that they know the facts behind the reported benefits to balance their decision. Many proponents of raw milk claim that industrially raised, antibiotic laden cattle given GMO corn feed produce milk that needs to be pasteurized because it is inherently of poor quality and unsafe, but that organically raised pasture fed cows produce milk that is safe. That is simply untrue. Pasteurization was developed in the mid 1800s to eliminate pathogenic and spoilage microorganisms, not to fix the problems of industrialized agriculture. There is no credible scientific evidence to support the suggestion that organic pasture-fed cows generate safer milk than cows from industrial farms. Contamination by pathogenic organisms comes from fecal matter, the environment, the handlers, packaging, storage, and undetected infections in the animals, and is unrelated to diet and housing conditions.
Additional claims such as that pasteurized milk causes allergies, asthma or other conditions are not supported by the scientific literature. The claim that raw milk is better for individuals who are lactose intolerant is not supported by scientific data either, and represents a clear misinterpretation or misunderstanding of available information. The level of nutrients in raw compared to pasteurized milk is not significantly different, invalidating yet another central claim in support of raw milk.
There is an abundance of misinformation on the topic of raw milk. Unfortunately, groups like Vassar’s Raw Milk Co-op perpetuate this misinformation. At Vassar, we often say “go to the source.” This is an opportunity to practice that principle. Rather than seeking confirmation of one’s beliefs in the websites of others, we must check the original research. What is actually supported by credible scientific investigations? Is the information you are reading being correctly interpreted? Are you getting the whole story or just fragments?
There are many good things about the locavore and Slow Food movements. Supporting small local farms and sustainable agriculture, humane treatment of animals and having the sense of community achieved from getting to know the farmer who raises your food, are important to me and many people. But you don’t need to consume raw milk to do that. These issues are distinct from the question of pasteurization. If you want, you can even get the raw milk and pasteurize it yourself. Just heat your milk on the stove to 63C for 30min before drinking it.
Thanks to the students in STS/Biol 172 for discussion and research on this topic
A few links:
FDA Milk Safety
Review on Milk Safety and Pasteurization
A recent review of the scientific literature. Note that they included many studies of poor design, which should have been ignored.
Slightly updated and edited on December 1, 2011
I Don’t Want Dengue Fever
When a student is absent from class, they usually send me an email to explain why. Occasionally I get emails from students in my microbiology or virology classes explaining their absence from class as a result of some infectious disease and they actually seem excited about the fact that they are hosting a virus. Perhaps they feel that they are participating in the class on a whole new level or are appreciating and understanding what is going on in their body, despite feeling awful. However, I was surprised recently when I mentioned Dengue Fever and a student piped up and said “I’ve had that!” I asked Caitlyn to write about her experience, and she kindly agreed. While she is interested in learning more about the virus, I suspect she would have preferred to learn about it without first hand experience. Here is her story.
Contributed by Caitlyn Anderson ’13
“I was infected with Dengue virus in Cambodia during the summer of 2007 while working as an intern for the Clinton Foundation. I knew before going that there was a Dengue epidemic across the country but was unwilling to give up the opportunity. It is likely that I was bitten by a mosquito carrying the virus while I was sight seeing in Siem Reap towards the end of my stay. The virus incubated within my body for a period of approximately 5 days. Thankfully, I was back on U.S. soil when the virus began to present itself. I remember feeling slightly odd as I worked the night shift at Starbucks. After I returned home I immediately went to bed. In the morning I had developed flu like symptoms with a fever of 100 degrees. My body began to feel achy and I remained in bed throughout the afternoon. By 3:00 pm my temperature had reached 103 degrees and by 5:00 pm, my temperature was up to 104 degrees and I could barely move. My mother immediately called my pediatrician who then instructed us to go to the Emergency Room. I had immense difficulties walking from my bed on the second floor to the car. When we got to Norwalk Hospital in Connecticut, I was unable to walk and required the assistance of a wheel chair. The initial reaction of the emergency room doctor who saw to me first was that I was presenting with Lyme like symptoms. However, the unbearable pain caused by the insertion of the IV into my arm was not indicative of Lyme disease so I was immediately admitted to the hospital for further tests and supportive care. A few hours later my fever had reached 105 degrees and was coupled with the sudden onset of rash covering my entire body. The virus began to affect my nervous system causing extreme skin tenderness. Infectious disease specialists were brought in to evaluate my case. A Haitian doctor was immediately convinced I had Dengue Fever because she had witnessed the disease many times. Unsure of which of the four strains I had been infected with, the doctors could not predict the clinical evolution of the disease.
My fever remained between 103 and 105 degrees for 3 days. I was treated with fluid intravenously and pain medication for my body aches and severe skin sensitivity. My body was packed with ice in an effort to lower my body temperature. While Dengue Fever is commonly referred to as “breakbone fever” because people often feel as if there bones are being crushed, I did not experience this sensation. My skin, rather than my bones and joints, was the greatest cause of my discomfort. On day 4 of my hospital stay, my fever began to go down to 100 degrees but I was transitioned to the telemetry unit so that my heart could be monitored more closely. I continued to receive IV fluids and pain medication. I remained in the telemetry unit until day 6 when I was moved to a general ward where I remained until my release from the hospital on day 8. My fever had completely dissipated but I was very weak and had trouble walking. When I returned home I slept for 16 hours a day for about a week and was able to return to school a few days later with a reduced academic schedule. About a month later I regained my strength was symptom-free.”
Does the Flu Vaccine Work?
There is a photo circulating on Facebook that shows the package insert for a flu vaccine that appears to indicate that the vaccine has not been shown to be effective against influenza. Of course, this has gone viral, (sorry for the pun) especially among the anti-vax crowd.
I wanted to do a little investigating to understand the statement on the package insert. The insert says: “FLULAVAL is a vaccine indicated for active immunization against influenza disease caused by influenza virus subtypes A and type B contained in the vaccine… This indication is based on immune response elicited by FLULAVAL, and there have been no controlled trials adequately demonstrating a decrease in influenza disease after vaccination with FLULAVAL”
Its the last statement that has triggered concern: no controlled trials adequately demonstrate a decrease of influenza disease after vaccination? Sounds bad, so lets take a look. The package insert actually has 14 pages. They show the results from a 2005-2006 clinical study involving 7482 people. About half received the vaccine and half received a placebo. Then they followed those individuals to see who got the flu. 23 people receiving the vaccine got a strain of flu against which the vaccine is supposed to protect. 45 receiving the placebo got those same strains of the flu. The statistical analysis shows a vaccine efficacy of about 46%, but the calculation of the confidence interval suggests the efficacy could be as low as 9.8%. Before doing the clinical study, they decided that the lowest limit of the confidence interval had to be above 35% to be considered successful. So it seems that the statement that clinical trials have failed to show efficacy is correct due to the large error range in their data.
Lets consider the other statement, that FLULAVAL is indicated based on it eliciting an immune response. Data from another study is shown in which people were given the vaccine and after 2 weeks, then checked for production of antibodies. In this study the levels of antibody increased to high enough levels in enough individuals that the vaccine met the criteria for success. Furthermore, they did a test called an Immunological Non-Inferiority test. Basically, they wanted to know if FLULAVAL induces an antibody response that is at least as good (ie not inferior to) another vaccine available on the market, FLUZONE. FLULAVAL induced as good a response as FLUZONE. (If you take a look at the FLUZONE package insert, they only report data on antibody responses, and state that no data is available on whether FLUZONE reduces incidence of influenza).
So there appears to be something of a conflict: the clinical trial was not successful but the vaccine appears to induce an appropriate response. Perhaps the measurement of antibodies is not the ideal indicator for predicting protection? This speaks to an important question in vaccine development, which is determining the correlates of protection. That is, what specific part of the immune response is needed for immunity?
Lets also look at other flu vaccines. FLULAVAL is one of seven different flu vaccines available.
Fluarix: Clinical studies show a reduction in influenza disease in vaccinated vs placebo groups.
FluMist: Clinical studies show a reduction in influenza disease in vaccinated vs placebo groups. The data for FluMist are the most impressive, getting as high as 96% efficacy with certain flu strains.
FluVirin: Only shows immunogenicity data, induces antibody response that exceeds the threshold defined for success.
FLUZONE: Only shows immunogenicity data, induces antibody response that exceeds the threshold defined for success.
Afluria: Only shows immunogenicity data, induces antibody response that exceeds the threshold defined for success.
Agriflu: Clinical studies show a reduction in influenza disease in vaccinated vs placebo groups.
Interestingly, it appears that approval of flu vaccines is based on showing that the vaccine can induce a strong antibody response, not showing that the vaccine prevents the disease.
Package inserts don’t communicate the whole story. We also have to consider the total body of evidence, not just one or two tests. There are many other clinical trials demonstrating the efficacy of flu vaccines. Such as this one, this one, this one, and this one.
In the clinical trials described in the package inserts, the severity of disease is not indicated. Did the vaccinated people get less severe disease than the non-vaccinated people? A vaccine that induces sufficient immunity so that it prevents severe disease although you might still get a sniffle, would still be pretty good. There are other outcomes to consider too. Does the vaccine reduce transmission or complications following influenza disease? In Canada, Ontario made efforts to dramatically increase influenza vaccination, with the result of reduced influenza associated mortality and reduced healthcare use. And take this study in which it was found that vaccination of healthcare workers didn’t reduce incidence of flu in those vaccinated but reduced the mortality rate of their patients.
There is an obvious need for a flu vaccine that induces better protection, especially in children and the elderly, and ideally, one that is universal so we dont have to go every year to get a shot and dont have to depend on predictions of what is going to circulate in the future. But the evidence that the flu vaccine is beneficial for individuals and society is pretty strong. Finally, I think this emphasizes the importance of digging deeper to understand the information around us. It is never as simple as it seems and we must avoid reducing information to the simplest single sentence thus removing the underlying complexities.
Disclaimer: I am “not that kind of doctor” so this is not intended to provide any medical advice or recommendations for which vaccine to use.





