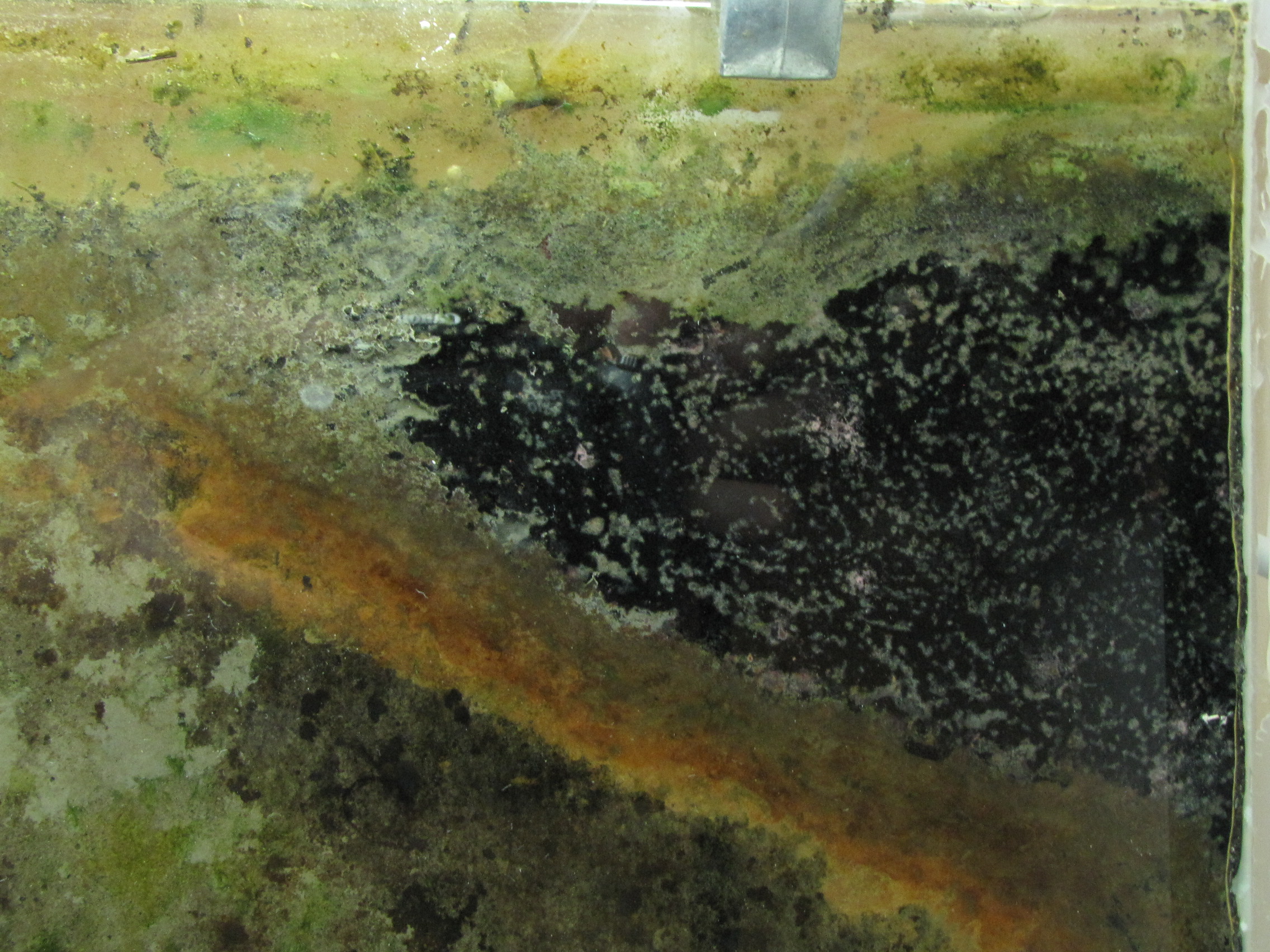
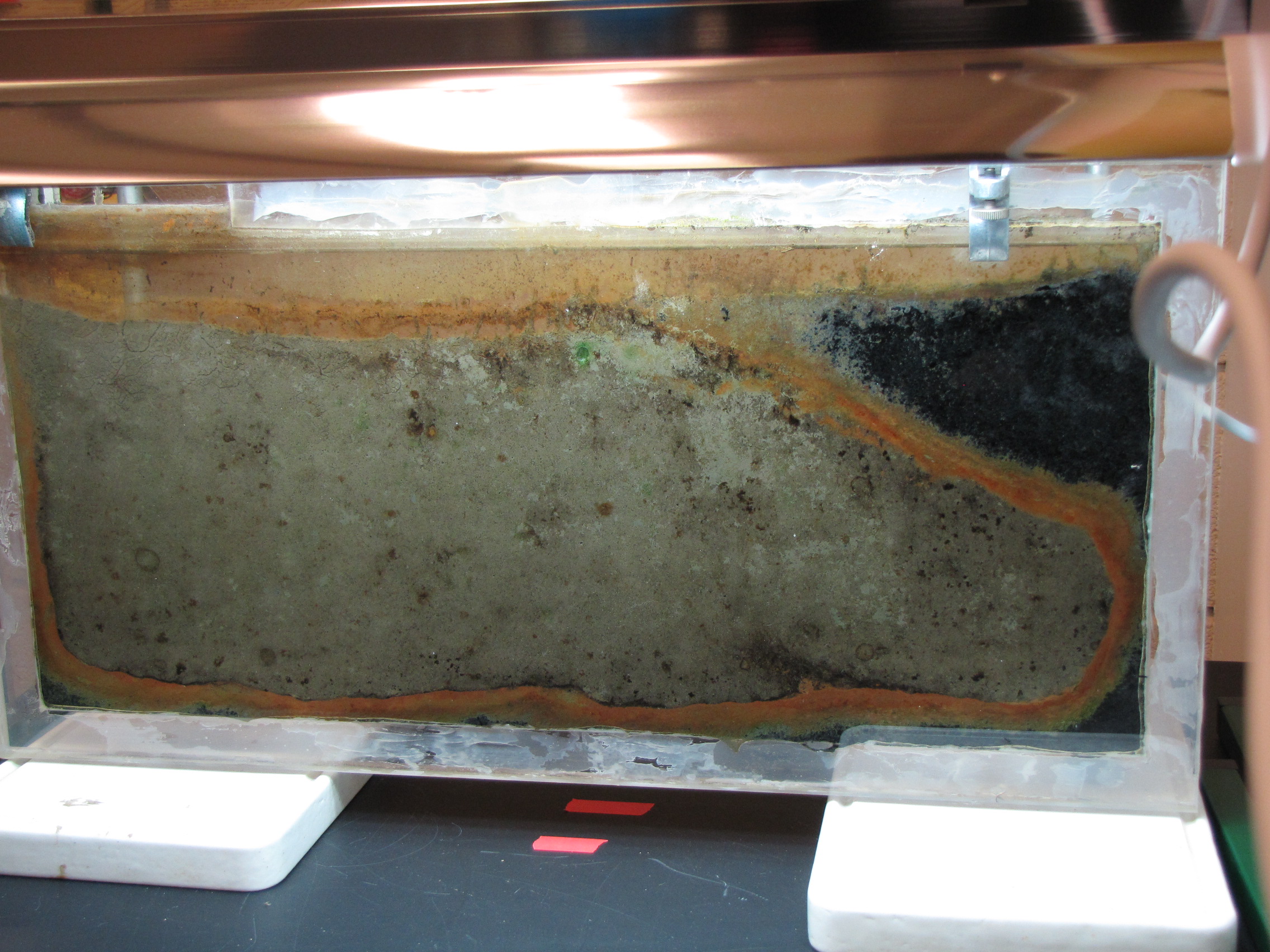
Temporal and Spatial Distribution of the Microbial Community of Winogradsky Columns
Winogradsky columns are model microbial ecosystems prepared by adding pond sediment to a clear cylinder with additional supplements and incubated with light. Environmental gradients develop within the column creating diverse niches that allow enrichment of specific bacteria. The enrichment culture can be used to study soil and sediment microbial community structure and function. In this study we used a 16S rRNA gene survey to characterize the microbial community dynamics during Winogradsky column development to determine the rate and extent of change from the source sediment community. Over a period of 60 days, the microbial community changed from the founding pond sediment population: Cyanobacteria, Chloroflexi, Nitrospirae, and Planctomycetes increased in relative abundance over time, while most Proteobacteria decreased in relative abundance. A unique, light-dependent surface biofilm community formed by 60 days that was less diverse and dominated by a few highly abundant bacteria. 67–72% of the surface community was comprised of highly enriched taxa that were rare in the source pond sediment, including the Cyanobacteria Anabaena, a member of the Gemmatimonadetes phylum, and a member of the Chloroflexi class Anaerolinea. This indicates that rare taxa can become abundant under appropriate environmental conditions and supports the hypothesis that rare taxa serve as a microbial seed bank. We also present preliminary findings that suggest that bacteriophages may be active in the Winogradsky community. The dynamics of certain taxa, most notably the Cyanobacteria, showed a bloom-and-decline pattern, consistent with bacteriophage predation as predicted in the kill-the-winner hypothesis. Time-lapse photography also supported the possibility of bacteriophage activity, revealing a pattern of colony clearance similar to formation of viral plaques. The Winogradsky column, a technique developed early in the history of microbial ecology to enrich soil microbes, may therefore be a useful model system to investigate both microbial and viral ecology.


 layer and anoxic sub-surface layers. Winogradsky columns have been used extensively to demonstrate microbial nutrient cycling and metabolic diversity in undergraduate microbiology labs. In this study, we used high-throughput 16s rRNA gene sequencing to investigate the microbial diversity of Winogradsky columns. Specifically, we tested the impact of sediment source, supplemental cellulose source, and depth within the column, on microbial community structure. We found that the Winogradsky columns were highly diverse communities but are dominated by three phyla: Proteobacteria, Bacteroidetes, and Firmicutes. The community is structured by a founding population dependent on the source of sediment used to prepare the columns and is differentiated by depth within the column. Numerous biomarkers were identified distinguishing sample depth, including Cyanobacteria, Alphaproteobacteria, and Betaproteobacteria as biomarkers of the soil-water interface, and Clostridia as a biomarker of the deepest depth. Supplemental cellulose source impacted community structure but less strongly than depth and sediment source. In columns dominated by Firmicutes, the family Peptococcaceae was the most abundant sulfate reducer, while in columns abundant in Proteobacteria, several Deltaproteobacteria families, including Desulfobacteraceae, were found, showing that different taxonomic groups carry out sulfur cycling in different columns. This study brings this historical method for enrichment culture of chemolithotrophs and other soil bacteria into the modern era of microbiology and demonstrates the potential of the Winogradsky column as a model system for investigating the effect of environmental variables on soil microbial communities.
layer and anoxic sub-surface layers. Winogradsky columns have been used extensively to demonstrate microbial nutrient cycling and metabolic diversity in undergraduate microbiology labs. In this study, we used high-throughput 16s rRNA gene sequencing to investigate the microbial diversity of Winogradsky columns. Specifically, we tested the impact of sediment source, supplemental cellulose source, and depth within the column, on microbial community structure. We found that the Winogradsky columns were highly diverse communities but are dominated by three phyla: Proteobacteria, Bacteroidetes, and Firmicutes. The community is structured by a founding population dependent on the source of sediment used to prepare the columns and is differentiated by depth within the column. Numerous biomarkers were identified distinguishing sample depth, including Cyanobacteria, Alphaproteobacteria, and Betaproteobacteria as biomarkers of the soil-water interface, and Clostridia as a biomarker of the deepest depth. Supplemental cellulose source impacted community structure but less strongly than depth and sediment source. In columns dominated by Firmicutes, the family Peptococcaceae was the most abundant sulfate reducer, while in columns abundant in Proteobacteria, several Deltaproteobacteria families, including Desulfobacteraceae, were found, showing that different taxonomic groups carry out sulfur cycling in different columns. This study brings this historical method for enrichment culture of chemolithotrophs and other soil bacteria into the modern era of microbiology and demonstrates the potential of the Winogradsky column as a model system for investigating the effect of environmental variables on soil microbial communities.