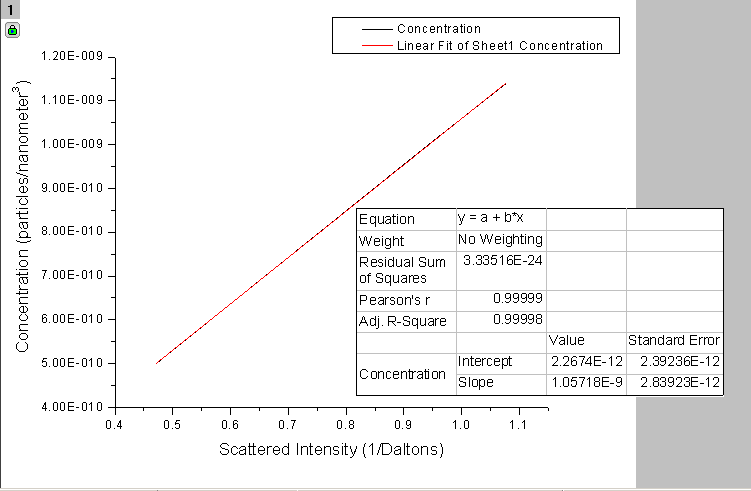
Origin Graph Depicting Scattering Intensity vs. Concentration
Using the theory of Static Light Scattering a graph was made for particle size 105 nm (in diameter) for volumes 1000, 2000, 3000, 4000 microliters and the predicted scattering intensity was found using Equation [3]. The wavelength used was 632.8 nanometers (red in the visible spectrum) so the particle size was within the Rayleigh limit. The scattering intensity was then plotted as the concentration of the particles was varied.
1/y-intercept was supposed to yield the molecular mass of a particle in Daltons. (1 Dalton=1amu). A linear fit was applied to the graph in order to find the y-intercept. As shown on the graph the y-intercept was found to be $2.3*{10^{-12}}$
which yields a molecular mass of approximately $4.5*{10^{11}} $ Daltons
In order to calculate the actual molecular mass of the microspheres the 2SPI website was used. Here’s the link: http://www.2spi.com/catalog/standards/microspheres.shtml
The approximate concentration listed on the website of the 0.105 micrometer spheres was ${10^{3}}$ particles/mL
The bead density was approximately $1.05$ grams/mL
which meant that each particle was approximately $1.05*{10^{-16}}$ kg
In order to convert between kilograms and Daltons it is important to note that $1.022*{10^{-27}}$ equals approximately 1 Dalton.
This yielded a molecular mass of $1.1*{10^{11}}$ Daltons
While the calculated molecular mass and the actual molecular mass are on the same scale (both ${10^{11}}$) the answer is still off by a factor of about 3 to 4.
This error may stem from the many approximations used when calculating the actual molecular mass. The number of particles/mL was estimated on the SPI website. Also the bead density was approximate. Since these polystyrene microspheres are mass produced it cannot be guaranteed that every particle is the exact same size and a perfect spherical shape. There will also be slight variations in the particle densities across different containers. However the particles need to meet certain standards, so the error is limited, but could possibly account for the small differences between the actual and calculated molecular mass.
In addition, since the slope was found to be $1.057*{10^{-9}}$ (which is greater than zero) it would appear, based on Static Light Scattering Theory, that the particles will stay in a stable solution. This information was confirmed on the 2SPI MSDS data and safety sheet for these microspheres (the sheet claims the solution formed with these sphere is stable).
Just for reference it seems like the method I used for calculating the molecular mass based on Static Light Scattering Theory is not the easiest, quickest, nor most accurate method. Equipment can be purchased that measures the intensity of the samples at different concentrations, compares it to a known standard, and outputs a Debye Plot which calculates the molecular mass and the gradient of the graph. However, it can be important to test theories in a more controlled method where every step of the process is known in order to check the accuracy of the theory and learn exactly how the equipment works.

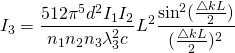







![Rendered by QuickLaTeX.com \begin{equation*} M_\delta = \dfrac{1}{\sqrt{2}}\left[ \begin {array}{ccc} e^{j\delta}&0\\ \noalign{\medskip} 0&e^{-j\delta} \end {array} \right] \end{equation*}](https://pages.vassar.edu/magnes/wp-content/ql-cache/quicklatex.com-e6eb01795d8c2f32c200ebdcdc2fb573_l3.png)
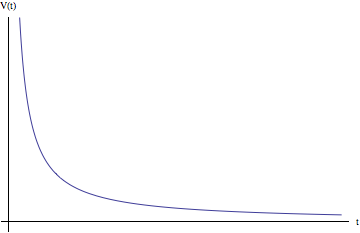
![Rendered by QuickLaTeX.com \begin{equation*} V = \frac{qc}{\sqrt{\left(c^2t-\frac{vz\sqrt{x^2+y^2+z^2}}{x^2+y^2+z^2}\right)^2 +\left(c^2-v^2\right)\left(x^2+y^2+z^2-c^2t^2\right)\right]}} \end{equation*}](https://pages.vassar.edu/magnes/wp-content/ql-cache/quicklatex.com-bb03d4a473db4f0166ae6687bb56e5f7_l3.png)