Animals living in natural ecosystems often live with the background noise produced by water features or insect calls, for example. This background noise can interfere with an organism’s abilities to effectively distribute their acoustic signals or perceive other acoustic signals which can be necessary for processes such as mating and hunting. For example, bats emit ultrasonic signals and use the returning echoes to gather information about prey during hunting. If the background noise is loud and overlaps with the spectrum of a signal, the signal becomes hard for an individual to disentangle, detect, and discriminate in a process called energy masking. Consequently, animals avoid such background noise or change their behavior in ways like making louder and longer calls. Background noise and animals’ responses to it can affect animal distributions, their ecology, and, in some cases, ecosystem services humans rely on.
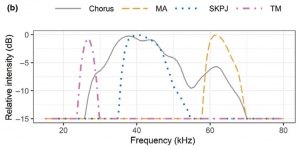
Figure 1b) Representative powerspectra of bat species’ echolocation calls relative to the katydid chorus playback, indicating the potential for acoustic masking. (From: Sedlock et al., 2021)
Sedlock et al. investigated whether meadow katydid (Conocephalus spp.) ultrasound choruses have an effect on bats that kill prey in the air (aerial hawking). They hypothesized that during the chorus, bats with sonar calls that most overlapped with the frequency of the katydid chorus (Scotophilus kuhlii and Pipistrellus javanicus –SKPJ) would be more likely to avoid the test area and alter their echolocation behavior due to energy masking.
To test their hypothesis, they played katydid call recordings on speakers in a katydid-free rice paddy and monitored bat activity using recorders and microphones that recorded the bat calls. They also accounted for prey availability by capturing and recording the number of arthropods flying in the test area.

Conocephalus spp. (From Candide Gardening)
Sedlock et al. found that their katydid chorus came with a decrease of SKPJ and MA activity within ~5 meters of the source (>/=75 dB threshold). (MA is Miniopterus australis and TM is Taphozous melanopogon: the other species of bats observed). Though not statistically significantly, SKPJ avoided the chorus more when closer to it, as did MA –to a lesser extent– but not TM. They also found that the number of arthropods collected was positively associated with SKPJ and TM activity except within the 75 dB/~5 m threshold.
Because of the observed change in bat activity near the chorus, researchers concluded that the insect noise can in fact influence bat activity. The decrease in activity during the chorus and close to the sound source by SKPJ and MA whose call frequency spectra overlap with that of the chorus suggests that energetic masking is impacting the bats’ echolocation. This conclusion is consistent with their hypothesis on avoidance. Researchers also added that the chorus indicating a poor-quality habitat may be a weaker, secondary mechanism behind the bat avoidance behavior. There were no data supporting their prediction that SKPJ would be more likely to modify their echolocating behavior as none of the bats appeared to alter their call.

From left to right, top then bottom: Scotophilus kuhlii, Pipistrellus javanicus, Miniopterus australis, Taphozous melanopogon. (From Ecology Asia, ResearchGate, Australian Museum, and Ecology Asia, respectively)
These results are important because they imply that katydid choruses could decrease bat habitats and cause domino effects in the ecosystem including an increased flying insect population, subsequent effects on plants, and reduced bat pest control. There are even potential impacts on our global food supply as katydid choruses are often found in rice paddies where bats eat the pests on the rice plants.
This study contributes to the field of sensory ecology by examining patterns and mechanisms of overcoming background noise. These findings can be applied to other contexts and organisms studied by researchers in the future. In the broad sense, studies like this can increase our understanding of the ways in which organisms affect each other and the impacts on the ecosystem scale and on human interests.
Reference:
Sedlock, J. L., Gomes, D. G. E., Rubin, J. J., Woody, S., Hadi, B. A. R., & Barder, J. R. (2021). A phantom ultrasonic insect chorus repels low-flying bats, but most are undeterred. Functional Ecology, 35(12), 2743-2752. https://doi.org/10.1111/1365-2435.13933
Image source links:
Conocephalus spp. https://candide.com/img/a321b930-2545-43c9-a25a-0e7fe31937e9/cropped/648×520
Scotophilus kuhlii https://www.ecologyasia.com/images-kl/lesser-asiatic-yellow-house-bat_7740.jpg
Pipistrellus javanicus https://www.researchgate.net/profile/Neil-Furey/publication/258243281/figure/fig3/AS:650874267385882@1532191926807/Pipistrellus-javanicus-CBC01255-Preah-Vihear-Protected-Forest-C-G-Csorba.png
Miniopterus australis https://media.australian.museum/media/dd/images/Miniopterus_australis.width-800.235878f.jpg
Taphozous melanopogon https://www.ecologyasia.com/images-ab/black-bearded-tomb-bat-cu_7150.jpg
