Every day humans are emitting carbon dioxide (CO2) into the atmosphere and causing problems to the environment. The first issue that comes to mind is always global warming and how the earth is going to melt away. I hope you realize by now that this information is false and that there are actual problems that are affecting the way our ecosystem functions.
One actual problem is the rising of carbon dioxide levels in the ocean. This disrupts the marine organisms and may impair the sensory systems and alter the behavior of marine fishes. How can one simple molecule such as CO2 cause all of this?
CO2 is a naturally occurring chemical compound that causes a decrease in the pH of Earth’s oceans, which is known as ocean acidification. When the pH drops, this also affects the fish’s ion balance and disrupts an important neurotransmitter in the brain called GABAA. GABAA is the chief inhibitory neurotransmitter in the central nervous system. Alterations to this inhibitory system can disrupt the visual system of a fish and delay the response time to a predator. This implies that the rise of CO2 levels in the ocean causes impairment in the vision of fish.
Chung et al. (2014) decided to study the affects of CO2 levels on visual responses in damselfish (Acanthochromis polyacanthus) in the Great Barrier Reef. To measure the visual response, Chung focused on vision at the retinal level and how the retina responses to a flickering light. He measured the critical flicker fusion (CFF) threshold of a fish which is the frequency at which light becomes continuous and the retina stops responding. Typically organisms that are fast moving and live in bright environments have higher CFF than organisms who spend most of their time in dark environments and are slow.
Chung and his colleagues measured the electrical light response of the retina in damselfish exposed to high levels of CO2 and high levels of CO2. The fish exposed to normal CO2 levels had a high CF of 90 Hz (frequency) while the fish exposed to the high CO2 levels after 6 days had a 68 Hz. This is a great prediction for the future to show if damselfish will have a harder time detecting fast moving objects.
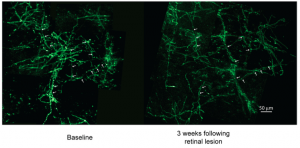
The experiment also tested whether increased CO2 levels in the ocean waters affected GABA signalling system. The GABA receptor was activated by treating the high exposure of carbon dioxide with gabazine As shown in Figure 1 below, the treatment restored the fish’s retinal performance and the CFF threshold increased.
 Overall the study showed that when a damselfish is exposed to high levels of CO2, their vision is impacted. However, there are no results indicating what happens in the ecosystem when their vision is impaired. We will need to perform more studies in the future to predict the predation and prey threat in the marine ecosystem
Overall the study showed that when a damselfish is exposed to high levels of CO2, their vision is impacted. However, there are no results indicating what happens in the ecosystem when their vision is impaired. We will need to perform more studies in the future to predict the predation and prey threat in the marine ecosystem
If you want to learn more about the methods and techniques of the experiment you can check out the latest article in the Journal of Experimental Biology.
Chung, W.S., Marshall N. S., Watson, S.A., Munday, P.L., Nilsson G.E. 2014 Ocean acidification slows retinal function in a damselfish through interference with GABAA receptor. J. Exp. Biol. 217: 311-312.