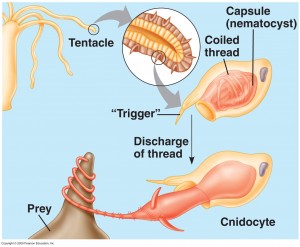
Cnidaria is a diverse phylum containing over 10,000 species, all belonging to two basic body forms: swimming medusae (jellyfish) and sessile polyps. These organisms employ a wide range of strategies for food acquisition ranging from filter feeding and symbiotic relationships with photosynthetic protists, to active prey capture with nematocyst laden tentacles (Fig. 1). While many Cnidarians using nematocysts for prey capture rely on opportunistic tentacle structures or swimming with the tentacles trailing behind to maximize the likelihood that a prey organism will come into contact with one of the tentacles, others have complicated visual systems thought to play a role in prey capture.
Figure 1. Some Cnidarians implement a nematocyst based prey capture mechanism as depicted above
A wide range of visual systems exists throughout various Cnidarians, from simple eye-spots to advanced pigment cups with lenses. However, the usefulness of complex visual systems had been called into question due to the apparent lack of neural branches to process the information the visual system receives or a nervous system to interpret visual images, instead simply possessing a decentralized nerve net. However, many Cnidarians do exhibit light mediated behavior.
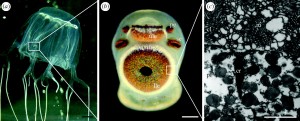
The most complex visual system of the phylum belongs to the class Cubozoa (Fig 2.). Carukia barnesi, a highly venomous Cubozoan have four sets of six ‘eyes’, which is typical among the class, consisting of a pair of simple light sensitive pigment cups, a pair of light sensitive pigment slits, and a pair of complex eyes that each have a cornea, a lens, and a retina. Behaviors that this visual system are thought to enable include: targeting light shafts for feeding, obstacle avoidance, actively swimming away from dark objects and decreased activity at night. However, the use of this visual system is prey capture is poorly understood. In their study, Courtney et al. (2015) aimed to describe part of the feeding ecology of the Cubozoan C. barnesi and understand the mechanisms employed by this species to capture its prey.
Fig 2. The visual structures of a Cubozoan
To go about their study, the researchers conducted several experiments to gain insight into the prey capture ecology of these organisms. First, they tested for a correlation between organism size and tentacle morphology and found a significant positive relationship between bell size and tentacle length but no correlation between bell size and distance between nematocyst clusters. Next, they tested the influence of light on tentacle extension and found that the tentacles were significantly longer in light environments than dark environments. Next, they tested the influences of bell-size, light and tentacle extension on “twitch-rates” of the tentacles and found larger bell-size, increased tentacle extension and increased light were all correlated with faster twitch-rates. Finally, they studied the interaction of C. barnesi and the larval fish they prey on and found that the fish were attracted to extended twitching tentacles.
These findings have important implications for our understanding of Cubozoan prey capture ecology, suggesting that these organisms are active predators with complex prey-capture mechanisms, capturing visually orientated prey by using a lure-like system to simulate the size and movements of the plankton the larval fish feed on. The larval fish are often visual hunters and therefore predominately feed during the day. This matches the finding that the luring behavior of C. barnesi does not occur in no-light conditions, most likely to conserve energy. Additionally, the widespread use of movement as a prey detection mechanism of larval fish suggests that the tentacle twitching utilizes movement of the nematocyst clusters to highlight these lures in the water column and increase catch rates.
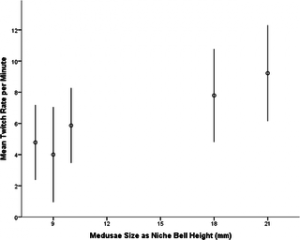
The finding that larger C. barnesi have increased twitch rates coincides with previous data that shows that during maturation C. barnesi undergo an ontogenetic venom change, correlated with a prey shift, from planktonic invertebrates to larval fish. The smaller organisms have a preference for plankton, which are most likely captured through a more passive prey capture seen in other Cnidarian medusae, “haphazardly encountering prey in the water column.” Therefore the twitching of tentacles, which presumably increases energy consumption, would be inefficient for the capture of small plankton, explaining the results shown in figure 3.
Figure 3. Twitch rates of C. barnesi of various sizes. Larger organisms exhibit faster twitch rates likely due to their prey preference.
In conclusion, C. barnesi have complex hunting strategies that require both the use of their own visual systems and the visual systems of their prey to maximize catch-rates and minimize unnecessary energy expenditure. Future work could look into coevolution of tentacle morphology and prey visual systems. It would be interesting to see if adaptions exist in the larval fish that enable them to differentiate the lure of C. barnesi from the plankton they feed on and how that could drive the evolution of predator, prey and the relationship between them.