Exciting things happened this week in the VAOL lab. For the first time this semester we were able to collect data on the worms.
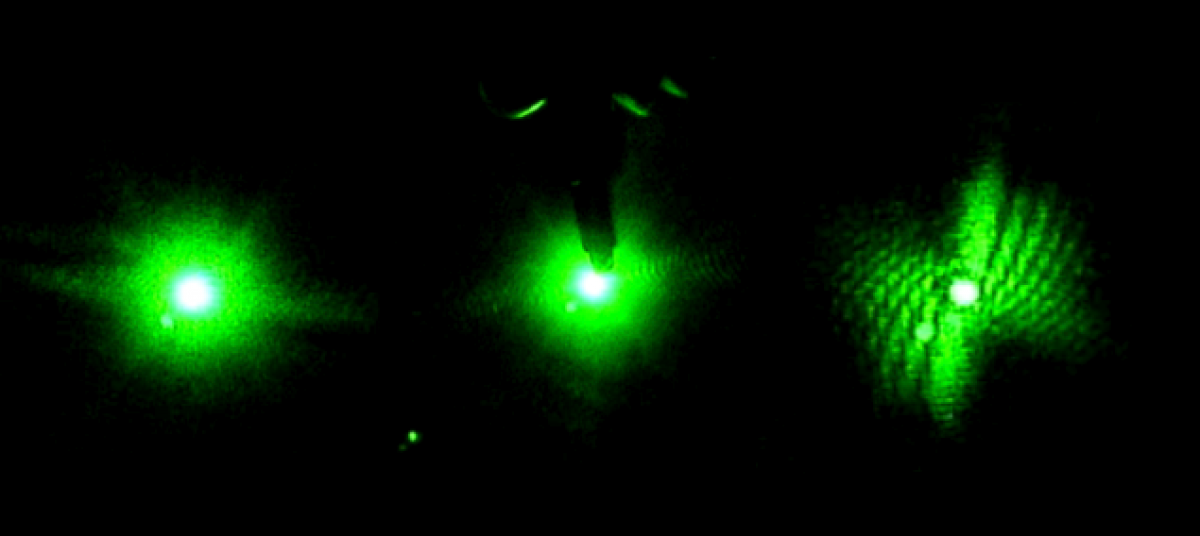
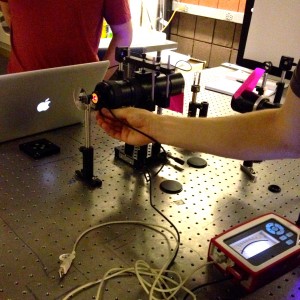
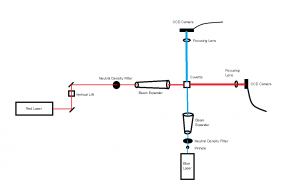
In the beginning of the week we spent time practicing transferring the worms from the cup they are grown in, into a cuvette filled with distilled water. This is more challenging than one would expect because the worms are microscopic and the process must be down under a microscope, using a tiny pick to lift the worms up and transfer them to the cuvette. We also practiced finding the worm once it was in the cuvette and getting the laser to pass through them to create a diffraction pattern. Once the worm is found in the cuvette with the naked eye it is pretty easy to keep the laser on it as it swims around.
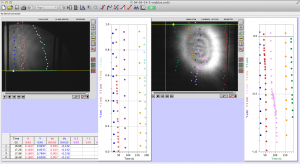
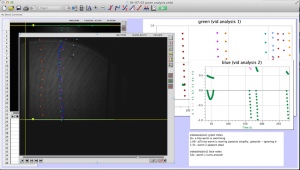
Later in the week, we actually collected data using the pico scope software on the computer. The pico scope evaluates the change in diffraction pattern observed by the photo diode as the laser passes through the worms. It then shows the results in voltage. We ran trials on four worms total, getting data for each one using only the red laser. The plan is to continue running data on more worms with both the blue and red lasers, after which we will combine all the data for analysis.
Things are finally starting to come together in the lab after what seemed like a lot of grunt work putting the lab together after the move.