The start to the second semester saw slow progression. With new experiments being held in the lab, we now have to share part of the lab table, as well as some of the equipment. Near the end of first semester we were having trouble with the table set up and had to change it to re-align the blue laser. We spent the first week of this semester cleaning up the lab and finishing what we had started with the table set up.
Next we had to adjust to the move of the biology lab from main building over to the renovated Olmsted hall. Although it is nice to be in the new labs, this took so time to adjust to because the supplies need for the worms was all over the place. We also had to gain card access to the building to access the lab on the weekends.
After we spent approximately the first two weeks finishing the lab set up we had to harvest a new culture of worms. Since Elias and I have different schedules this semester, and the fact that he is devoted more time to the worms this semester, we decided to harvest two plates of worms. This works better because we can now run data on worms three days a week, depending on the life cycle of the worms as they are on a four day life cycle. We run data on the fourth day of the cycle to keep consistence throughout all the trails.
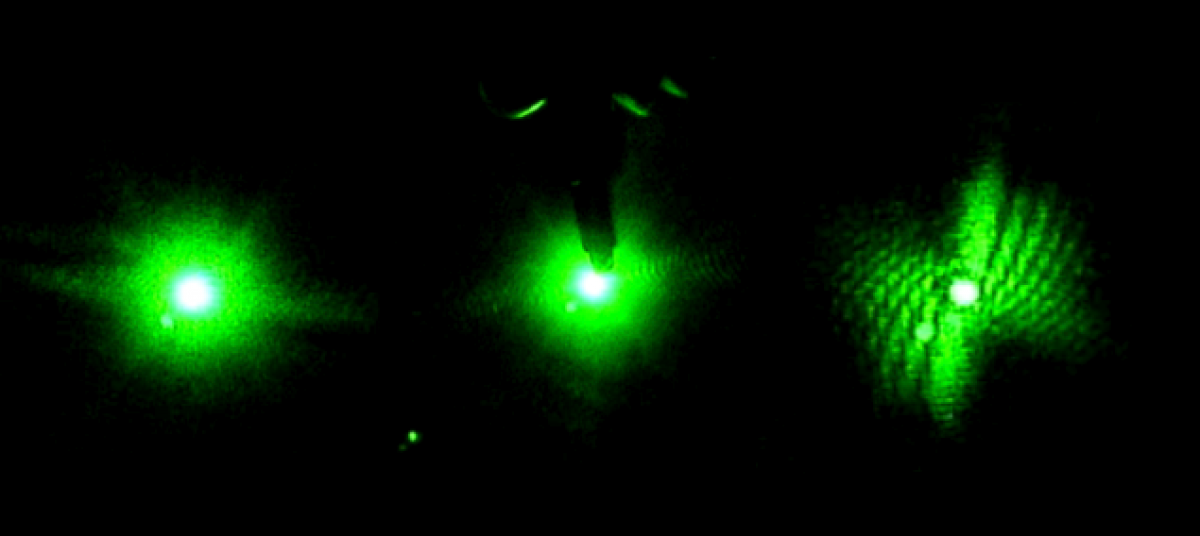
Next it was on to starting to run data. We realized that last semester we were having problems with too much ambient light coming into the lab space from the outside windows. To fix this we repositioned the curtain in the lab to better block out the light. This helps get a better reading on the true thrashing frequency of the worm because there is less disturbance from outside light.
This semester, with added practice and a better set up we have become much more efficient with our time in the lab. We have been running a lot of data on the worms twice a week. However at times, it is still frustrating that we don’t get as much done in the lab each week as we may like. Since I am only spending 4 hours a week in the lab, the whole semester is equivalent to the amount of time spent in the lab over one week during the summer. It is difficult to get a lot done in some of the sessions because some of the time is spent harvesting the worms, and readjusting the table alignment if it was disturbed.
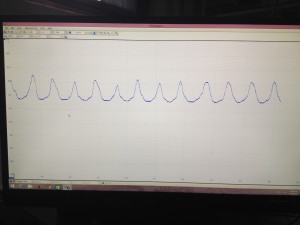
We have also developed a solid procedure to analyze the data using MatLab. We have a code that we insert the data from the Picoscope into MatLab and it analyzes the frequency. This frequency is equivalent to the thrashing frequency of the C. elegans. We have begun to gather a large amount of analyzed data, and are working to hopefully get enough data to better the consistency of our results.