Cassandre Stirpe ’15 (Collins Fellow)
In icy and snowy winter conditions, de-icing salt (primarily NaCl) helps to keep paved roads and walkways safe for both drivers and pedestrians, but this benefit is offset by detrimental environmental impacts. Salt can pollute local streams and soils, and at high enough concentrations becomes harmful to living organisms.
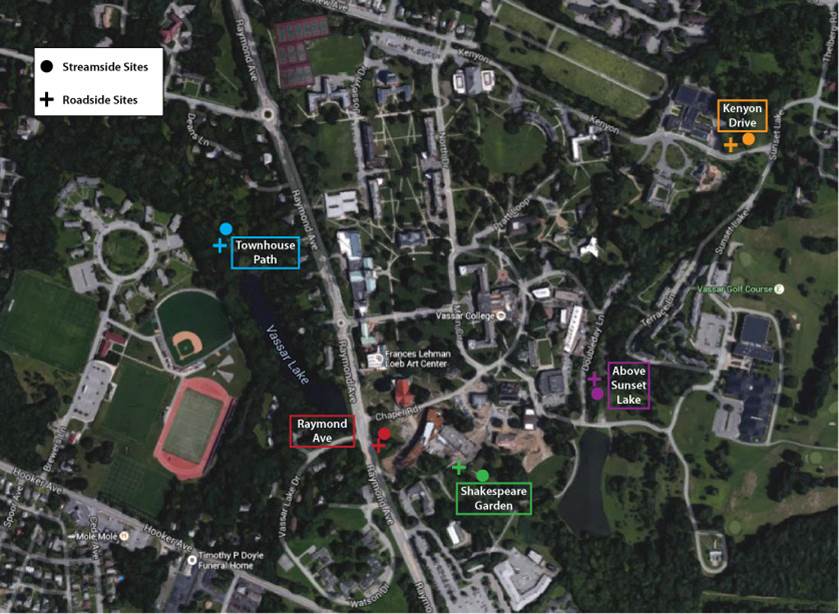
This study sampled soils on Vassar campus, both near paved roads and walkways, and along the local Fonteynkill and Casperkill streams. The goal of the study was to see whether campus soils were retaining salt, and how the impacts differed between sites that were near the road, and sites downslope and further away.
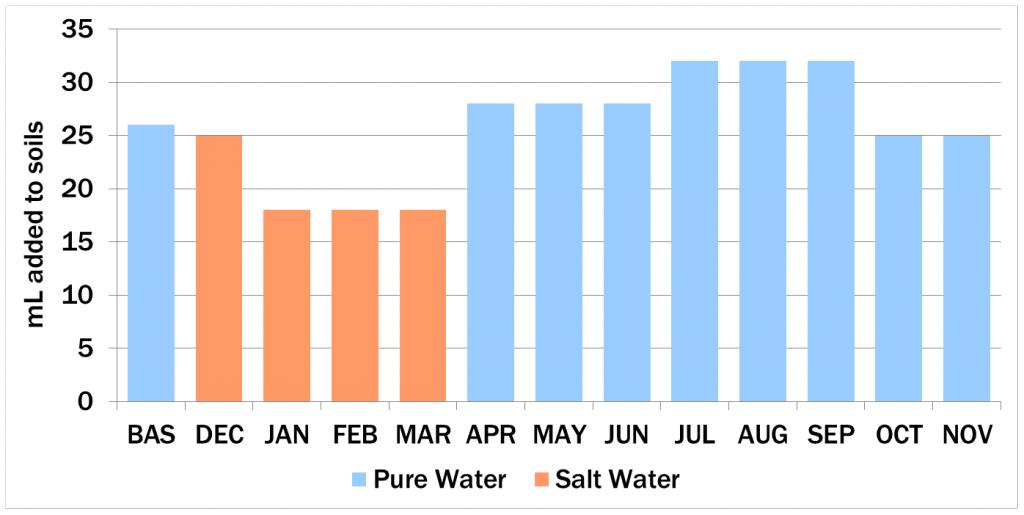
Ten soil samples were collected (one for each marked location) and studied by running water (either pure water or salt water) through them and collecting the resulting filtrate. The goal of this was to simulate a year of precipitation and salting.
During the simulated year, chloride concentrations increased during the simulated months December, January, February, and March, when salt water was added to represent snow and the coincident salting activity. After salting ceased, fresh water applications flushed chloride out of the soils again. Most flushing occurred in the first couple of treatments (April, May, June, July), with removal rates tapering off in simulated autumn. Roadside sites were flushed more quickly than streamside sites, and seem to have had all chloride removed. Streamside sites showed a slight delay, due to lower permeability. They were also more likely to retain chloride at the end of the experiment.
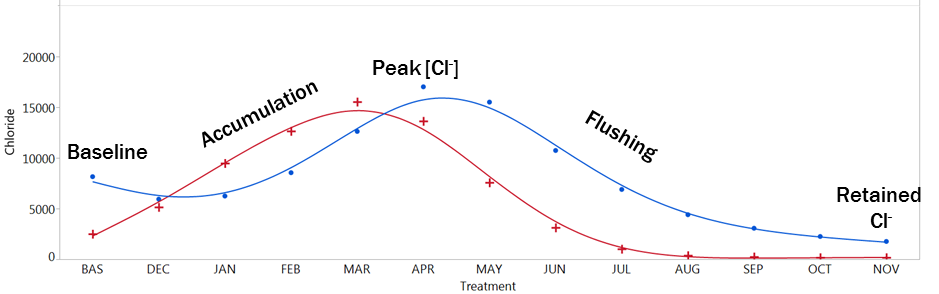
Over the course of the study, chloride concentrations increased as salt was added, then decreased as soils were rinsed with fresh water. Salt concentrations peaked in simulated March-April, with streamside sites (blue) flushing chloride more slowly than roadside sites (red).
The ion concentrations of the soil filtrate indicate that de-icing salt has negatively affected both roadside and streamside soils.
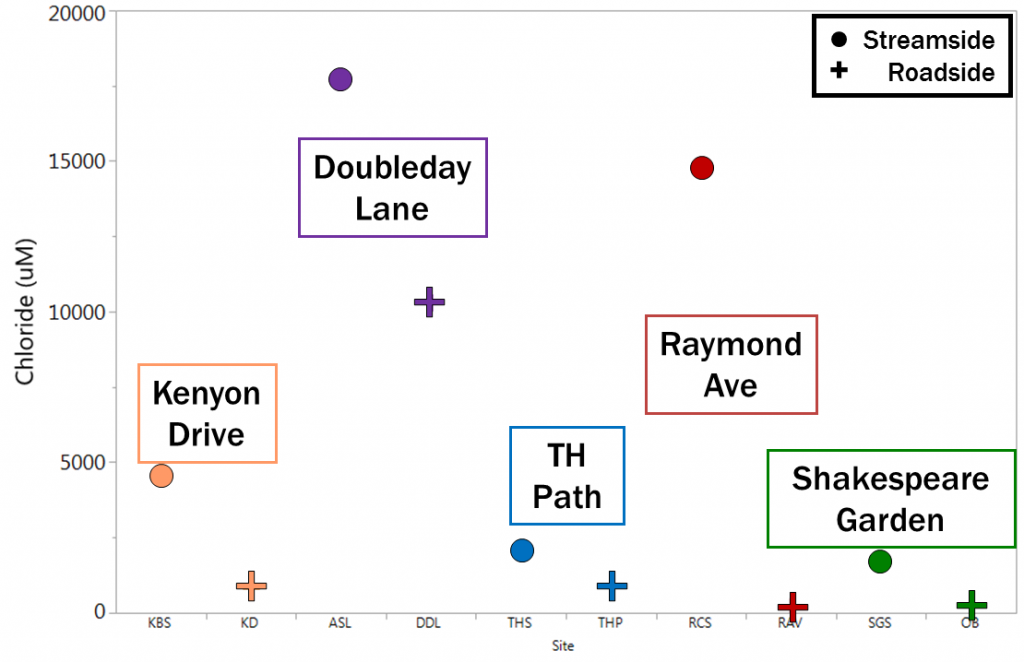
Baseline chloride concentrations (taken at the beginning of the experiment) reveal that streamside sites had greater amounts of accumulated chloride than their roadside counterparts. This indicates that the chloride has traveled downslope, away from roadside sites, and is accumulating in the clay-rich streamside soils.
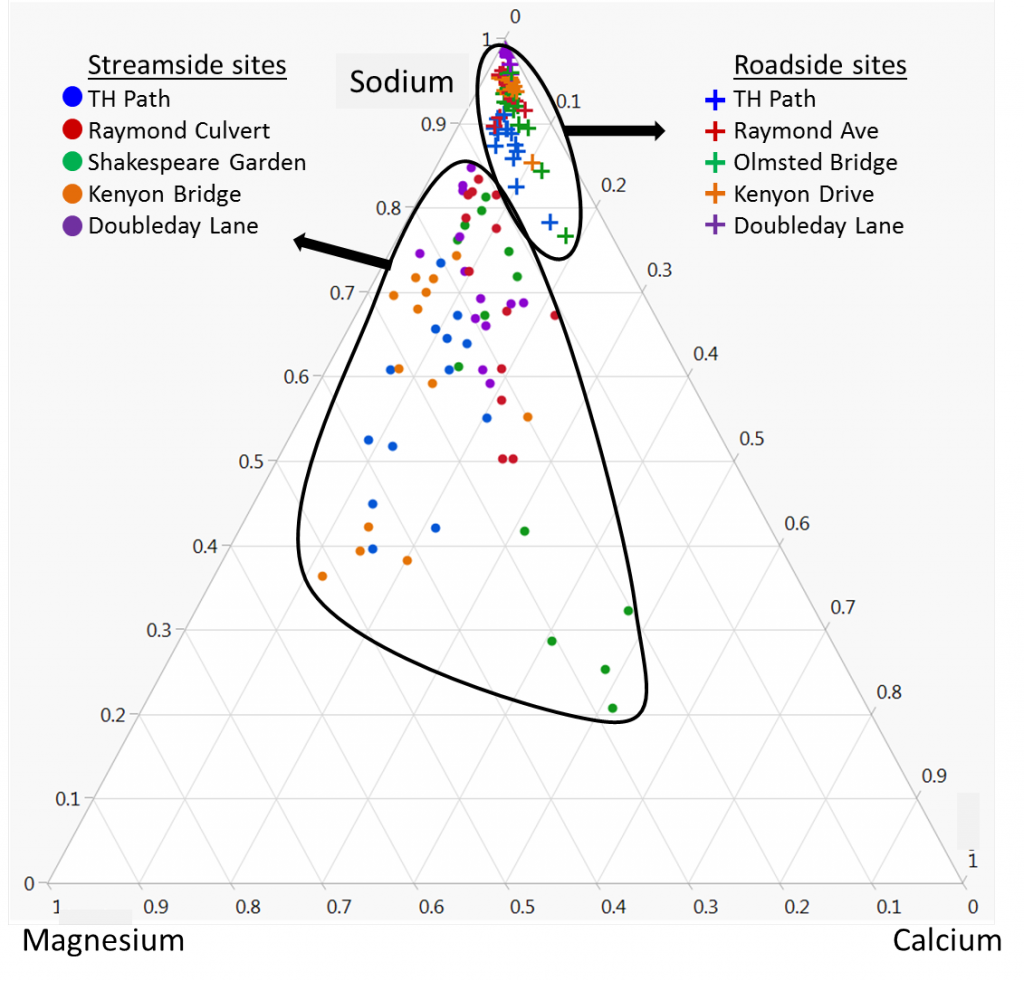
Cation composition also varied dramatically between streamside and roadside sites. Roadside sites contained mostly sodium, while magnesium and calcium (both important for plant growth) had been leached from the soils. Streamside sites showed more variability and contained more magnesium and calcium. This indicates that sodium is displacing calcium and magnesium at roadside sites.
This study concludes that both roadside and streamside soils have been negatively affected by road salt (NaCl), but they have been affected in different ways. Streamside soils are accumulating chloride that has traveled downslope, while roadside soils are accumulating sodium and losing other cations which are displaced and leached away.
Due to the many roads and pathways on Vassar campus that are salted each year, it is likely that most soils are campus are negatively affected in one way or another. In some areas, perhaps soil degradation is regarded as unimportant–but given the number of recent landscape plantings on Vassar campus, salt toxicity should be considered a concern.