Last official update!
This week, along with taking more data, we also took a cursory look at all the videos so far and simply counted how many worms “turned around” or “hovered” during the data collection. This was just to take a step back and get a sense for what rough results we were getting as to whether the worms have a sensitivity to different wavelengths of light. The results of that:
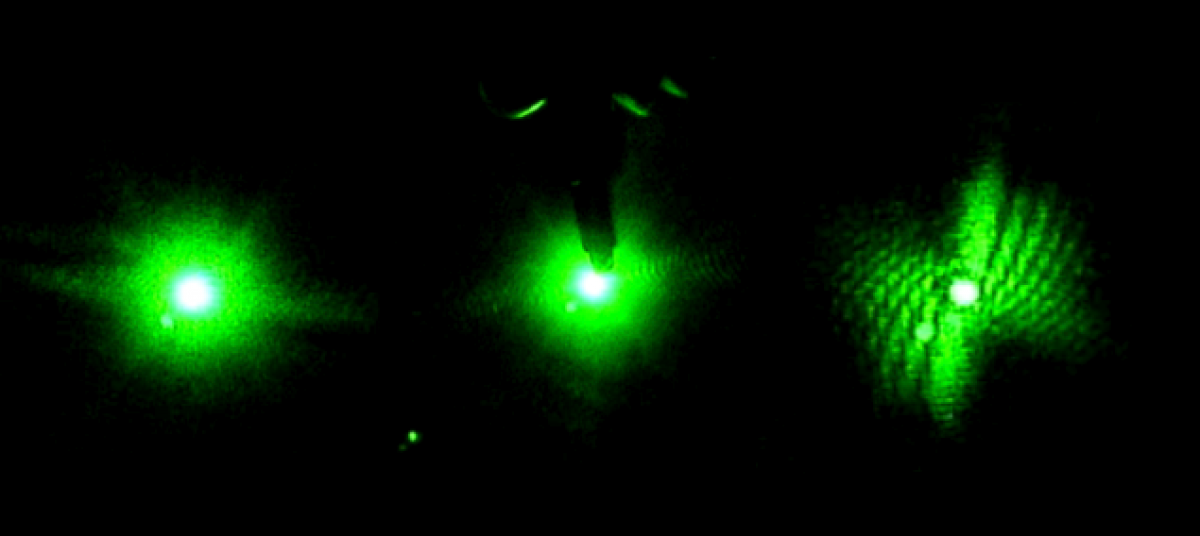
Red and Blue Lasers
Live Videos:
04-04-01 red – 0:52 hovering
04-04-03 blue – 1:16 hovering
Dead Videos:
04-04-01 blue – 3:00 extreme deceleration (the worm is dead though, so probably due to currents)
Green and Blue Lasers
Live Videos:
04-07-01 green – 3:45 (video 1) slight turnaround (maybe due to a current)
04-07-02 green blue video – 0:52 (video 2) turnaround
This data is from 6 (or so) total videos. This data hints that the worms may have a sensitivity to the different wavelengths of light, but is inconclusive so far.
Again, this week we took data with the blue and red lasers.
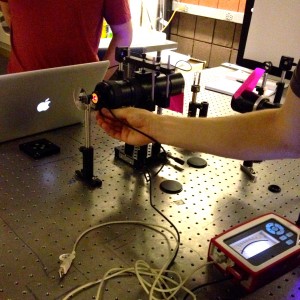
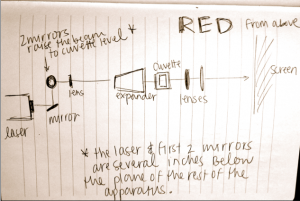
This image features the beam expander for the red laser, and Brian testing the power of the red laser. It is necessary for the two lasers to be producing the same power output for the experiment to be valid. (Note the setup: in view in order from left to right is the: neutral density expander, the power gauge held by Brian, the beam expander, the tiny cuvette, then the little black square of the CCD camera, and the white screen.)
This semester of research has been so eye-opening and informative to me personally and I feel that it is important to reflect briefly. I have learned through experience that experimental research is not smooth sailing: it requires ingenuity and patience facing the constant barrage of small problems in need of a solution. It is also quite rewarding and easy to invest in– keeping in mind the overall goal of the project, it is fun and easy to fall into the small details and celebrate every small victory. I have found this very rewarding, and am quite excited to continue in the field of research.