The research being pursued by the Tanski group is generally in the field of transition metal coordination chemistry. Research projects are available for interested students in several fields, including asymmetric catalysis, extended structure coordination chemistry, inorganic medicinal chemistry, and X-ray crystallography.
Undergraduate students at any level may receive credit or workstudy pay for time spent on research projects. See the bottom of this page for information on students currently working in the Tanski lab.
Major Research Projects:
1. Gold Coordination Chemistry
“Synthesis, structural characterization and in vitro anticancer screening of several gold(I) thiolate, dithiocarbonate and dithiocarbamate complexes;” Liu, H.; Tanski, J. M. Polyhedron, 2022, 115999.
2. Small Molecule X-ray Crystallography
Students work with Dr. Tanski, and other faculty members, to determine the crystal and molecular structures of compounds produced in our laboratories here at Vassar – both in coursework and in research – and also those from labs of our collaborators in academia and industry.
–> We are interested in detailed descriptions of molecular structures, including describing different types of intermolecular interactions, such as hydrogen bonding, π-stacking, halogen-halogen interactions, and C-H•••X (X = O, N, halogen) interactions. “π-stacking” should probably be regarded as geometrical arrangement instead of an intermolecular interaction: Some examples:
“Spectroscopic, crystallographic, and Hirshfeld surface characterization of nine-membered-ring-containing 9-methoxy-3,4,5,6-tetrahydro-1H-benzo[b]azonine-2,7-dione and its parent tetrahydrocarbazole;” Flores, M. J.; Mai, B.; Tanski, J. M. Acta Cryst. 2023, E79, 831-836.
“Crystallographic and spectroscopic characterization of two 1-phenyl-(1H)-imidazoles:4-(1H-imidazol-1-yl)benzaldehyde and 1-(4-methoxyphenyl)-1H-imidazole;” McClements, I. F., Wiesler, C. R., Tanski, J. M. Acta Cryst. 2023, E79, 678-681.
“π-Stacking Motifs in the Crystal Structures of Bis(phosphine) Copper (I) η2-Tetrahydroborate Complexes;” Lueckheide, M.; Rothman, N.; Ko, B.; Tanski, J. M. Polyhedron 2013, 58, 79-84.
“Analysis of Small Molecule X-ray Crystal Structures: Chemical Crystallography with Undergraduate Students in a Teaching Laboratory;” Aldeborgh, H.; George, K.; Howe, M.; Lowman, H.; Moustakas, H.; Strunsky, N.; Tanski, J. M. J. Chem. Cryst. 2014, 44, 70-81.
“Crystal structures of 4-chloropyridine-2-carbonitrile and 6-chloropyridine-2-carbonitrile exhibit different intermolecular π-stacking, C-H···Nnitrile and C-H···Npyridine interactions;” Montgomery, M. J., O’Connor, T. J., Tanski, J. M. Acta Cryst.2015, E71, 852-856.
“Two Achiral Isomers of Chloronitropyridine Crystallize as Polar Materials with Different Molecular Packing Motifs Based on Similar Intermolecular Interactions;” Merritt, H.; Tanski, J. M. J. Chem. Cryst. 2018, 48, 109-116.115.
–> For details on our analysis of other metrical parameters such as pitch angle, see:
“Structural Analysis of Bisphenol-A and its Methylene, Sulfur, and Oxygen Bridged Bisphenol AnalogsLim, C. F.; Tanski, J. M. J. Chem. Cryst. 2007, 37, 587-595.
–> We are also interested in absolute structure, particularly of organic compounds:
”Aza-Crown Macrocycles as Chiral Solvating Agents for Mandelic Acid Derivatives;” Quinn, T. P.; Atwood, P. D.; Tanski, J. M.; Moore, T. F.; Folmer-Andersen, J. F. J. Org. Chem. 2011, 76, 10020-10030.
“Structural Studies of Diaminocyclohexane-Containing Aza-Crown Macrocycles and Their Zn(II) Complexes;” Atwood, P. D.; Akyant, A.; Shalumova, T.; Tanski, J. M.; Folmer-Andersen, J. F. Polyhedron 2014, 67, 191-198.
“Crystallographic and spectroscopic characterization of (R)-O-acetylmandelic acid;” Cirbes, C.; Tanski, J. M. Acta Cryst. 2016, E72, 901-903.
Vassar has a Bruker APEX DUO X-ray diffractometer.
3. Asymmetric Lewis Acid Catalysis
Multiple asymmetric induction in homogeneous catalysis by metal coordination complexes with various different combinations of chiral ligands is an attractive approach to enhancing enantioselectivity in existing catalytic processes. A specific example of such an application is the methylation of aryl aldehydes with dimethyl zinc catalyzed by Lewis acidic metal coordination complexes of chiral alkoxides and a chiral chelating ligand, L*.
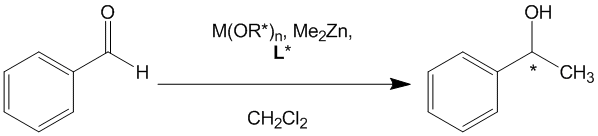
In contrast to the more well studied and optimized process of ethylation with diethyl zinc, methylation results in significantly lower enantiomeric excess. We have recently shown that it is possible to modestly increase the enantiomeric excess of 1-phenylethanol obtained from the methylation of benzaldehyde by dichiral double asymmetric induction with a titanium(IV) complex of commercially available BINOL (BINOL = 1,1’-bi-2-naphthol) and the resolved chiral sec-butoxides Ti(OR-2Bu)4 or Ti(OS-2Bu)4. (MacMillan, S. N.; Ludford, K. T.; Tanski, J.M. Tetrahedron: Asymmetry2008 , 19, 543–548.) The simple resolved titanium sec-butoxides, combined with resolved BINOL of the opposite configuration designation, yielded a matched pair that mediates the addition of dimethyl zinc to benzaldehyde(>99% conversion) with higher enantioselectivity (~58%) than resolved BINOL with Ti(OiPr)4 (~46%) or the mismatched pair (~40%). We also investigated another chiral chelating ligand, tetrahydrosalen. (MacMillan, S. N.; Jung, C. F.; Shalumova, T.; Tanski, J. M. Inorg. Chim. Acta 2009, 362, 3134-3146.)
We were later able to achieve even higher enantiomeric excesses of 80-90% using a different chiral diol, R,R-TADDOL, although we found that the matched pair effect is less prominent with R,R-TADDOL than it is with R– or S-BINOL. We have most recetnly observed that with modified, chlorinated TADDOL ligands, we can achieve >99% conversion to the methylated product with 96.5% enantiomeric excess. (Baker-Salisbury, M. G.; Starkman, B. S.; Frisenda, G. M.; Roteta, L. A.; Tanski , J. M. Inorg. Chim. Acta 2014, 409, 394-398.)
We have more recently turned our attention to synthesizing chiral substituents on amines by the reduction of imines.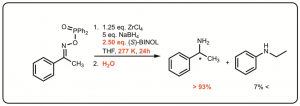
 URSI 2022
URSI 2022
4. Covalent Metal Organic Networks
Metal-organic coordination network (MOCN) materials formed from rigid organic spacers and metals of known coordination tendencies have become increasingly well studied. Materials with large pores present possibilities for:
- molecular recognition,
- separation, and
- catalytic transformations
of guest molecules. In addition, chiral nanoporous materials are an emerging area of research in this field. The vast majority of networks are formed from later transition metals and rigid carboxylate or pyridine based organic spacer ligands. A key feature of MOCN materials is that considerable structural predictive ability exists over traditional solid-state inorganic compounds in their design.
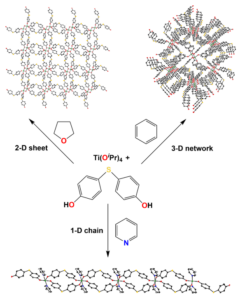
We have studied a unique class of early transition metal covalent metal-aryloxide coordination polymers synthesized from bisphenolic spacer precursors and titanium(IV) alkoxides. Depending on the nature and geometry of the precursor and the donating ability of the solvent used in the reaction, network structures of diverse dimensionality and topology may be obtained. The reaction of 4,4’-bis(hydroxyphenyl)sulfide with Ti(OiPr)4 yields three different network materials depending on the solvent:
VSI Dedicated to Professor Peter T. Wolczanski on the occasion of his 70th birthday.
“Solvent control of dimensionality in titanium aryloxide coordination polymers prepared from 4,4’-bis(hydroxyphenyl)sulfide;” Lim, C. F.; Ludford, K. T.; Tanski, J. M. Polyhedron 2024, 116860.
Laboratory:
A laboratory with equipment for the synthesis of air and water sensitive inorganic and organometallic compounds is in Bridge 207. The lab is fully equipped with two 4-port vacuum/inert gas lines and a gloveboxe. Reactions are carried out using both Schlenk and swivel-frit techniques.
Student researchers:
Sean Majer (Chemistry ’15) and Rob Tureski (Chemistry ’14)
Tanski Research Group (2008-10)

From left: Prof. Tanski, Nyanza Rothman (Chemistry ’11), Leslie Roteta (Chemistry ’10), and Tamila Shalumova (Chemistry ’11)
Tanski Research Group (2007-08)

From left: Grace Tan (Biochemistry ’09), Marina Hitosugi-Levesque (Biochemistry ’09), Christine Jung (Biology ’09), William Jobs (Chemistry ’10), Tamila Shalumova (Chemistry ’11)
Tanski Research Group (2006-07)

From left: Caitlin Lim (Chemistry ’07), Mike Zubrow (Biology ’07), Andre Dennis (Chemistry ’06), Kaysia Ludford (Chemistry ’07) and Sam MacMillan (Chemistry ’07)
URSI 2009
From left: Leslie Roteta (Chemistry ’10) and Tamila I. Shalumova (Chemistry ’11)
URSI 2008

From left: Greg Maier (Chemistry ’09), Grace Tan (Biochemistry ’09), Gabrielle Phillip (Biochemistry ’09), Tamila Shalumova (Chemistry ’11)
URSI 2007
Grace Tan (Biochemistry ’09) and Marina Hitosugi-Levesque (Biochemistry ’09).
URSI 2006
Kaysia Ludford (Chemistry ’07)
URSI 2005
Kaysia Ludford (Chemistry ’07) and Andre Dennis (Chemistry ’06)
URSI 2004
Andre Dennis (Chemistry ’06) and Donnele Daley (Physics ’06)
Former student researchers:
Class of 2004:
1. Christine M. Phillips, Ph.D. in biochemistry with Prof. Drennan at MIT, UC Berkeley post-doc, now Christine Phillips-Piro, Associate Professor of Chemistry at Franklin & Marshall College.
Class of 2006:
2. Donnele Daly, Vassar physics major in The Dual Degree Program with Dartmouth University’s Thayer School of Engineering, 2011 M.D. Penn State College of Medicine, general surgery resident, NYU School Of Medicine, New York.
3. Andre Dennis, graduate studies in inorganic chemistry with Prof. Eichhorn at the University of Maryland, College Park, now a Digital Marketing Professional in New York, NY.
4. Michael Zubrow, 2011 M.D. The School of Medicine at Stony Brook, resident at Dartmouth-Hitchcock Children’s Hospital at Dartmouth.
Class of 2007:
5. Caitlin F. Lim, D.O.; M.S. in Biomedical Sciences, 2008, Boston University, finsihed DO Philadelphia College of Osteopathic Medicine.
6. Kaysia T. Ludford, 2014 M.D, M.A. in biochemistry with Prof. Carroll at the University of Michigan, M.D. Yale School of Medicine, residency at Brigham and Women’s Hospital, Boston.
7. Samantha N. MacMillan, 2013 Ph.D. in inorganic chemistry with Prof. Peters at MIT & CalTech, postdoc in the Department of Chemistry and Chemical Biology at Cornell University with Prof. Lancaster, now the Director of the X-ray Facility in Cornell’s chemistry department.
8. Philipose Mulugeta, 2011 M.D. Vanderbilt University School of Medicine.
Class of 2009:
9. Greg Maier, graduate student with Prof. Butler in inorganic/bioinorganic chemistry at UC Santa Barbara.
10. Grace Tan, research technician at Weill Cornell Medical College (Rockefeller University), currently graduate student in Molecular and Cellular Pharmacology with Prof. Seeliger in the Department of Pharmalogical Sciences, Stony Brook University School of Medicine.
11. Marina Hitosugi-Levesque, MPH (Health Policy and Management) University of Hawai’i (Manoa), MD at John A. Burns School of Medicine at University of Hawai’i, resident in LA, Fellow in Geriatrics at the University of Hawaii .
12. Christine Jung, 2013 M.D. Chicago Medical School (Rosalind Franklin University), resident in emergency medicine, Boston Medical Center.
Class of 2010:
13. Leslie Roteta, Research Assistant at Mount Sinai School of Medicine, currently graduate student in the Interdisciplinary Graduate Program in Biomedical & Biological Sciences at Vanderbilt University School of Medicine.
Class of 2011:
14. Tamila Shalumova, did some graduate studies in chemistry at Stanford University.
Class of 2012:
15. Mollie Baker-Salisbury (Biochemistry) dual MD/MA Urban Bioethics student at the LKSOM (Temple).
Class of 2013:
16. Brian Starkman (Biochemistry) currently attending medical school at the State University of New York Downstate Medical Center College of Medicine.
Class of 2014:
17. Rob Tureski, currently in chemistry graduate school at UC Davis.
18. Edith Iyer-Hernandez
Class of 2015:
19. Sean Majer, graduate school in chemistry at Cornell.
Class of 2016:
20. Haley Merritt, graduate school in chemistry at NYU.
21. T.J. O’Connor, graduate school in inorganic/organometallic chemistry at UC Berkeley.
Class of 2017:
22. Mary-Margaret McElduff, QA/QC Technician at Yards Brewing Company in Philadelphia.
23. Bufan Zhang, graduate school in chemistry at Rochester University.
Class of 2018:
24. Austin Atsango, graduate school in chemistry at Stanford.
25. Liat Kugelmass, graduate school in chemistry at Cornell.
26. Yun Young Kim (Biochemistry), Research Assistantship at NIH, now MD/PhD program at Stonybrook.
Class of 2019:
27. Jaimeson Bukacek-Frazier
28. Rachel Josef, Dartmouth Engineering 2020.
29. Bevan Whitehead
30. Molly Youse, Medicinal and Pharmaceutical Chemistry grad school at Purdue University.
Class of 2020:
31. Henry Liu
Class of 2021:
32. Kevin Horiszny
33. Susanna Monroe, graduate school in chemistry.
Class of 2022:
34. Lucy Kuhn, Research Technician at Harvard Medical School, pre-med.
35. Brandon Mai
Class of 2023:
36. Hector Contreras, graduate school in chemistry at Wesleyan.
37. Isobelle McClements, graduate school in chemistry at Cornell.
38. Emma Sagerer
Class of 2025:
39. Maritza Flores

Christine Phillips (now Phillips-Piro) (Chemistry ’04) and Samantha MacMillan (Chemistry ’07) in 2008, while at the Chemistry graduate program at MIT











![IMG_0604[1]](http://pages.vassar.edu/jotanski/files/2012/01/IMG_06041-300x225.jpg)

