It is sad to say that this will be the last post for the academic year; however I have enjoyed working in the VAOL with Brian, Tewa, and Lily and would like to do so again in the future. For the last week of classes we spent a last bit of time analyzing the test videos that we took to ensure the equipment was set up properly and then began the testing that would happen in large amounts for huge sets of raw data.
Brian and I went through the small amount of test videos and quickly recorded anything that the worms did that wasn’t falling normally under gravity.
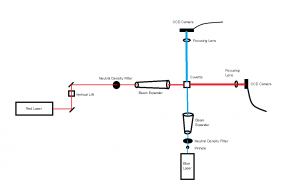
In the Red and Blue laser set up a worm hovered in the red light at 52 seconds in video 04-04-01, and another worm hovered at 1 minute 16 seconds in the blue light in video 04-04-03. One odd occurrence happened in the videos of dead worms at 3 minutes in the blue light a worm decelerated at an extreme amount which I also believe was caused by a current.
In the Green and Blue laser set up we noticed a worm make a slight turn around at 3 minutes 45 seconds in green light in video 04-07-01, but a current seemed to be the cause as well. However, in video 04-07-02 at 52 seconds a wormed turned around of it’s own accord.Overall, the data seems to suggest that some worms are reacting to the change in light; however, it is not an extremely consistent habit for them to attempt escape from the blue light. Given more time I would like to test the worms under the change into the beam of a UV laser which was mentioned in previous posts. The testing that we began used the same set up and the same methods during the test videos except we set the frame rates on each camera to be the same so that the video speed would remain consistent while analyzing the data in logger pro.