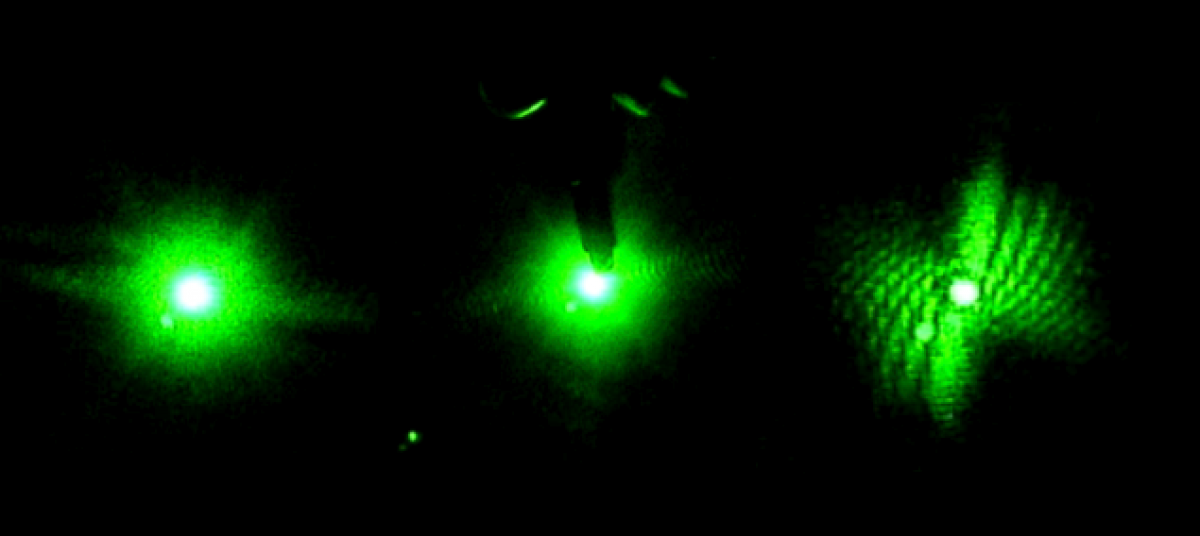
In the lab, things do not go smoothly line to line like in classroom physics problems. Experiments take endurance and patience: you will fail before you succeed.
Current project: Analyzing the C. elegans’ reaction to different light wavelengths (blue and red).
Problem: The videocamera cannot effectively record the nematode’s path through both the red and blue– it is very hard to track its path when analyzing the data.
Proposed Solution: CCD Camera.

A “charge-coupled device” camera, although much pricier than a digital camera, converts the light it senses into electrons (just like a solar cell). They then interpret the assembled charge, and transfer the analog data into digital pixels. These cameras produce extremely high-quality images due to the charge moving across the chip with very little distortion.
This particular camera (pictured) is great for the setup because it can 1: be placed directly in the beam to collect images, and 2: plugs into the computer to easily record the images.
What’s next? First we have to confirm that this CCD camera will solve the above problem. Then onto data collection: it is unknown whether the worms react more to green or blue light. It is known that they swim away from UV light. First, collect data for blue and green. Then, on to a UV laser?