Communication is vital to the survival of many species as it facilitates various critical aspects of social interaction, including territorial establishment and reproduction. Attention is a significant facet of communication as an individual receiving the signal, or information, must be actively “listening” to the emitter of the signal in order to effectively receive the information. Predation has a significant influence on these signals as it usually directly influences the noticeability of the signals, as the emitter’s signal is optimally adapted to prevent the predator from localizing the emitter. This decreased noticeability is often caused by a behavioral alteration to the production of the signal, such as a change in the amount of time an emitter spends signaling/what time of day is spent signaling or an alteration to the physical properties of the signal. However, there is usually a trade-off in this decreased noticeability as it lowers the signal’s efficiency in drawing the attention of an intended receiver. These behavioral changes taking place, in order to lower the noticeability of signals, are the focus of many studies today.
Steinberg et al (2014) focused on this notion of behavioral changes, specifically lowering the noticeability, in the mating signal of the male brown anole, Anolis sagrei, in response to predation by its main predator, Leiocephalus carinatus.

An image of a male Anolis sagrei. (http://upload.wikimedia.org/wikipedia/commons/thumb/9/94/Anolis_sagrei.jpg/1024px-Anolis_sagrei.jpg)
A. sagrei generally communicate, signaling territory or mating, through visual displays , including “head bobbing” and dewlap, the colorful flap on their throat, expansion/retraction. Furthermore, previous work has found/suggested that the head bobbing aspect of their communication is used in order to gain the attention of inattentive receivers. In addition, the intensity of the head bobbing has also been found to positively correlate with the maximum distance in which the signal can be effectively detected by the receiver, which is also referred to as the “active space” of the signal. This active space influences the ability and range of each male to repel other males and attract females.
(An example of both head bobbing and dewlap extraction/retraction in the anole lizard. )
Previous research has found that the predation of A. sagrei by L. carinatus has caused A. sagrei to alter their behavior, for example they have been found to avoid the ground and spend most of their time up in vegetation in the presence of this predator. L. carinatus are also a visually-dependent species that use head bobs, therefore the authors of this study hypothesize that the presence of this predator will influence the head-bob signal, through selective pressure, of A. sagrei. They predicted that it would lead to a decrease in the noticeability, or amplitude, of this signal and a lower amount of time dedicated to signaling.
In order to test their hypothesis, the researchers monitored and recorded the behavior, specifically the amplitude and proportion of head-bobs throughout the day, of 9 different populations (each on a different island) of brown male anoles. There were control groups which lacked the presence of L. carinatus, and there were experimental groups with varying numbers of L. carinatus present. The results of their study stated that there was no difference in the amount of time spent head-bopping between the control and experimental groups. However there was a significant difference in the amplitude between these groups, with the experimental groups displaying a significantly lower amplitude than that of the control groups.
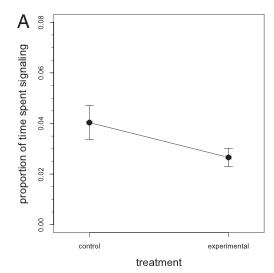
Figure 2 of the paper displaying the lack of a significant difference in the proportion of time spent signaling between the control and experimental groups.
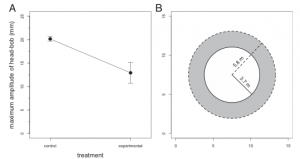
Fig 3 (A) of the study depicts the significant difference in the amplitudes of head bobbing between the control and experimental groups. With the control group displaying a greater amplitude of head bobbing. And Part (B) conveys the assumed difference in active space between the control and experimental groups.
Finally, the researchers concluded that the adjustment, or modulation, of visual signals by A. sagrei in the presence of L. carinatus further provides support for this concept of the influence of the selective pressure of predators on the signals of certain species. Furthermore, they emphasize the importance of this modulation as it can be immediately applied by a single individual and can be scaled, or slightly altered, based on the control/goals of the individual at that moment. They suggest that this lack of difference between the control/experimental groups when it came to proportion of time spent signaling may be due to the greater importance of signaling over the risk of being located by a predator, or may be due to the lack of a significant benefit (when taking into account the significant benefit from the alteration of amplitude) that would come about from adjusting signaling time.
References:
Steinberg, D.S., Losos, J.B., Schoener, T.W.,Spiller, D.A., Kolbe, J.J., & Leal, M. (2014) Predation-associated modulation of movement-based signals by a Bahamian lizard. Proceedings of the National Academy of Sciences of the United States of America. 11(25), 9187–9192.
